Mean Molecular Weight The thermodynamic relations between P, ρ
... equation. In general, the Saha equation can be used to compute ionization fractions over most of the star. It does, however, require that the gas be in thermodynamic equilibrium. This is true throughout the star, as at high densities, collisions will control the level populations. This approximation ...
... equation. In general, the Saha equation can be used to compute ionization fractions over most of the star. It does, however, require that the gas be in thermodynamic equilibrium. This is true throughout the star, as at high densities, collisions will control the level populations. This approximation ...
Chemistry Lab 2016-2017 Thermodynamics and Gases
... Which greenhouse gas has the most direct contribution to the greenhouse effect? A. Ozone B. Methane C. Carbon dioxide D. Water vapor Which property is the same for two samples of two different gases at the same temperature? A. Conditions of triple point B. Number of molecules C. Average molecular ve ...
... Which greenhouse gas has the most direct contribution to the greenhouse effect? A. Ozone B. Methane C. Carbon dioxide D. Water vapor Which property is the same for two samples of two different gases at the same temperature? A. Conditions of triple point B. Number of molecules C. Average molecular ve ...
Unit 9 Review
... Mathematically, this means that their _______________ is a constant. 12. The _______________ Gas Law permits calculation of any one term when temperature, pressure, and volume change for a gas. 13. If A and B are directly proportional and the value of A becomes 1/3 as much, what happens to the value ...
... Mathematically, this means that their _______________ is a constant. 12. The _______________ Gas Law permits calculation of any one term when temperature, pressure, and volume change for a gas. 13. If A and B are directly proportional and the value of A becomes 1/3 as much, what happens to the value ...
Chemical reactions occur with outer level electrons so that is the
... Aluminum has 3 valence have 10 electrons and 13 protons…… That’s +13 – 10 = +3 Charge ...
... Aluminum has 3 valence have 10 electrons and 13 protons…… That’s +13 – 10 = +3 Charge ...
Atomic Structure: 1. The smallest particle of an element that retains
... What is the number of electrons in the outermost energy level of an oxygen atom? ...
... What is the number of electrons in the outermost energy level of an oxygen atom? ...
Quiz 3 Feedback Electron Jumps in Atoms Emission and absorption
... “You get the temperature of a star by looking to see whether it has emission lines, absorption lines, or a continuous spectrum.” •All stars essentially have absorption lines on a thermal (continuous) spectrum. If there are emission lines, those come from low-density hotter gas above the surface. “Yo ...
... “You get the temperature of a star by looking to see whether it has emission lines, absorption lines, or a continuous spectrum.” •All stars essentially have absorption lines on a thermal (continuous) spectrum. If there are emission lines, those come from low-density hotter gas above the surface. “Yo ...
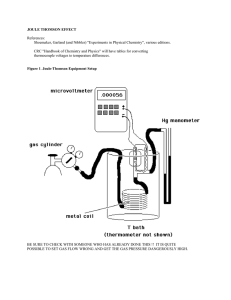
Final Exam for Physics/ECE 176 Professor
... 2. If the size L of a macroscopic cubic nonequilibrium system is increased by a factor of 2 and the largest temperature difference ∆T across the system is decreased by a factor of 1/2 (without changing the physical properties of the system), then the relaxation time time τ will (a) remain unchanged. ...
... 2. If the size L of a macroscopic cubic nonequilibrium system is increased by a factor of 2 and the largest temperature difference ∆T across the system is decreased by a factor of 1/2 (without changing the physical properties of the system), then the relaxation time time τ will (a) remain unchanged. ...
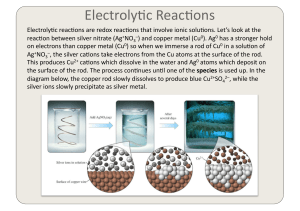
Electrochemistry 2
... generate Fe2+ ions. If we connect a metal that is more easily oxidised, electrons will flow from the easily oxidised metal to the point of strain and prevent the forma)on of the Fe2+ ions. Magnesi ...
... generate Fe2+ ions. If we connect a metal that is more easily oxidised, electrons will flow from the easily oxidised metal to the point of strain and prevent the forma)on of the Fe2+ ions. Magnesi ...
Factsheet
... __________________________ 41. __________________________ __________________________ 42. __________________________ ...
... __________________________ 41. __________________________ __________________________ 42. __________________________ ...
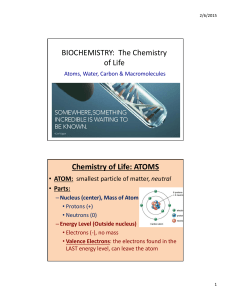
BIOCHEMISTRY: The Chemistry of Life Chemistry of Life: ATOMS
... • Atoms have an EQUAL number of protons and electrons • Ions have an UNEQUAL number of protons and electrons, have a CHARGE – Cation: more protons than electrons, POSITIVE charge, LOSE electrons – Anion: more electrons than protons, NEGATIVE charge, GAIN electrons ...
... • Atoms have an EQUAL number of protons and electrons • Ions have an UNEQUAL number of protons and electrons, have a CHARGE – Cation: more protons than electrons, POSITIVE charge, LOSE electrons – Anion: more electrons than protons, NEGATIVE charge, GAIN electrons ...
Distribution of alkali gases in Io`s atmosphere
... sodium are known to be present in Io's neutral clouds and plasma torus, which are believed to be fed from the moon itself.The immediate sources of gaseous NaCl and KCl in Io's atmosphere are still unknown. Based on thermochemical arguments, both molecules could be present in volcanic plumes. Their l ...
... sodium are known to be present in Io's neutral clouds and plasma torus, which are believed to be fed from the moon itself.The immediate sources of gaseous NaCl and KCl in Io's atmosphere are still unknown. Based on thermochemical arguments, both molecules could be present in volcanic plumes. Their l ...
Microplasma

Microplasmas are plasmas of small dimensions, ranging from tens to thousands of micrometers. They can be generated at a variety of temperatures and pressures, existing as either thermal or non-thermal plasmas. Non-thermal microplasmas that can maintain their state at standard temperatures and pressures are readily available and accessible to scientists as they can be easily sustained and manipulated under standard conditions. Therefore, they can be employed for commercial, industrial, and medical applications, giving rise to the evolving field of microplasmas.