Quantum Theory of Atoms and Molecules
... Electrostatics and Wave phenomena. Coulomb’s law, charge, electric field, electrostatic potential, dipole moment, polarisability; simple harmonic motion, forced oscillations, resonance; standing waves, travelling waves, transverse waves, longitudinal waves; the wave equation. ...
... Electrostatics and Wave phenomena. Coulomb’s law, charge, electric field, electrostatic potential, dipole moment, polarisability; simple harmonic motion, forced oscillations, resonance; standing waves, travelling waves, transverse waves, longitudinal waves; the wave equation. ...
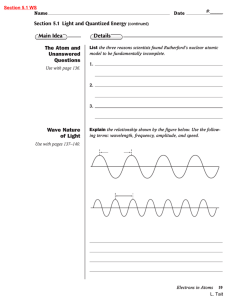
Ch. 5.3 study guide
... 10. The energy of a body can change only in small discrete units. 11. The position and velocity of an electron in an atom can be determined with great certainty. 12. The photoelectric effect will occur no matter what frequency of light strikes a metal. ...
... 10. The energy of a body can change only in small discrete units. 11. The position and velocity of an electron in an atom can be determined with great certainty. 12. The photoelectric effect will occur no matter what frequency of light strikes a metal. ...
Slide 1 - s3.amazonaws.com
... 7.4 Quantum Mechanics Physicists were both mystified and intrigued by Bohr’s theory. They questioned why the energies of hydrogen electron are quantized, or, why is the electron in a Bohr atom restricted or orbiting the nucleus at certain fixed distance? For a decade there is no logical explanation ...
... 7.4 Quantum Mechanics Physicists were both mystified and intrigued by Bohr’s theory. They questioned why the energies of hydrogen electron are quantized, or, why is the electron in a Bohr atom restricted or orbiting the nucleus at certain fixed distance? For a decade there is no logical explanation ...
Quantum Theory and Electrons as Waves
... If light could have particle-like behavior, then could matter have wave-like behavior? ...
... If light could have particle-like behavior, then could matter have wave-like behavior? ...
Quantum Mechanics Lecture Course for 4 Semester Students by W.B. von Schlippe
... world, the world of atoms and molecules and of atomic nuclei and elementary particles. The need for a revision of the foundations of mechanics arises as a result of the wave-particle duality of matter, which manifests itself in systems of atomic dimensions. Wave-particle duality means that particles ...
... world, the world of atoms and molecules and of atomic nuclei and elementary particles. The need for a revision of the foundations of mechanics arises as a result of the wave-particle duality of matter, which manifests itself in systems of atomic dimensions. Wave-particle duality means that particles ...
The Quantum Mechanical Model of the Atom
... particles, then particles should be able to behave like waves. The wavelength for particles should be: ...
... particles, then particles should be able to behave like waves. The wavelength for particles should be: ...
Quantum Mechanics as dissolver of the sensate universe: this is
... sources produce interference patterns; yet in 1839, it was first shown that light waves falling on metal caused the emission of electrons, which suggests that light has particle properties. Louis de Brogli generalized wave particle duality by associating a wave length not only with mass-less photons ...
... sources produce interference patterns; yet in 1839, it was first shown that light waves falling on metal caused the emission of electrons, which suggests that light has particle properties. Louis de Brogli generalized wave particle duality by associating a wave length not only with mass-less photons ...
Quantum Mechanics
... light was a wave (Newton thought it was a particle, but Young disproved his theories) According to wave theory, it should not depend on color of light, but rather the intensity Einstein determined that the photoelectric effect is in fact proof that light is a photon (particle) and has energy bas ...
... light was a wave (Newton thought it was a particle, but Young disproved his theories) According to wave theory, it should not depend on color of light, but rather the intensity Einstein determined that the photoelectric effect is in fact proof that light is a photon (particle) and has energy bas ...
Lecture 1
... world, the world of atoms and molecules and of atomic nuclei and elementary particles. The need for a revision of the foundations of mechanics arises as a result of the wave-particle duality of matter, which manifests itself in systems of atomic dimensions. Wave-particle duality means that particles ...
... world, the world of atoms and molecules and of atomic nuclei and elementary particles. The need for a revision of the foundations of mechanics arises as a result of the wave-particle duality of matter, which manifests itself in systems of atomic dimensions. Wave-particle duality means that particles ...
Quantum Theory of Light. Matter Waves.
... Modern and Classical Physics Classical physics treats particles and waves as different aspects of the reality. However, the physical reality arises from small-scale world of atoms and molecules, electrons and nuclei. Electrons behave as particles because they have charge and mass, but moving electr ...
... Modern and Classical Physics Classical physics treats particles and waves as different aspects of the reality. However, the physical reality arises from small-scale world of atoms and molecules, electrons and nuclei. Electrons behave as particles because they have charge and mass, but moving electr ...
Postulate 1
... something in common – wave properties. This led Schrodinger, in particular, to wonder whether equations used to describe light waves could be modified to describe the behaviour of electrons. This led to quantum mechanics and a probabilistic description of the behaviour of electrons. ...
... something in common – wave properties. This led Schrodinger, in particular, to wonder whether equations used to describe light waves could be modified to describe the behaviour of electrons. This led to quantum mechanics and a probabilistic description of the behaviour of electrons. ...
Synopsis
... Don’t try to work out the momentum of a high speed particle and hence its de-Broglie wavelength using its rest mass. Mas increase significantly as the speed of the particle approaches the speed of light! ...
... Don’t try to work out the momentum of a high speed particle and hence its de-Broglie wavelength using its rest mass. Mas increase significantly as the speed of the particle approaches the speed of light! ...