Welcome to Physics 112N
... Classical physics can describe the shape of the blackbody spectrum only at long wavelengths. At short wavelengths there is complete disagreement. This disagreement between observations and the classical theory is known as the ultraviolet catastrophe. ...
... Classical physics can describe the shape of the blackbody spectrum only at long wavelengths. At short wavelengths there is complete disagreement. This disagreement between observations and the classical theory is known as the ultraviolet catastrophe. ...
Topic 15
... •One of the fundamental consequences of quantum mechanics is that it is IMPOSSIBLE to SIMULTANEOUSLY determine the POSITION and MOMENTUM of a particle with COMPLETE PRECISION •Can be illustrated by a “thought experiment” known as Heisenberg’s Microscope, using radiation of wavelength λ to “look at” ...
... •One of the fundamental consequences of quantum mechanics is that it is IMPOSSIBLE to SIMULTANEOUSLY determine the POSITION and MOMENTUM of a particle with COMPLETE PRECISION •Can be illustrated by a “thought experiment” known as Heisenberg’s Microscope, using radiation of wavelength λ to “look at” ...
Chapter 4: Electrons in Atoms I. Properties of Light A
... 2. EM radiation are forms of energy which move through space as waves a. Move at speed of light (c) (1). c= 3.00 x 10^8 m/s b. Speed is equal to the frequency times the wavelength c = νλ (1). Freqency (ν) is the number of waves passing a given point in one second, measured in Hz or s^-1 (2). Wavelen ...
... 2. EM radiation are forms of energy which move through space as waves a. Move at speed of light (c) (1). c= 3.00 x 10^8 m/s b. Speed is equal to the frequency times the wavelength c = νλ (1). Freqency (ν) is the number of waves passing a given point in one second, measured in Hz or s^-1 (2). Wavelen ...
Chapter 13 – Electrons in Atoms
... Atoms/elements emit light when the electrons are excited (first absorb then emit energy in the form of light) at specific frequencies. ...
... Atoms/elements emit light when the electrons are excited (first absorb then emit energy in the form of light) at specific frequencies. ...
final2012
... a) What orbitals are filled for the protons and neutrons in these two nuclei? List how many neutrons or protons are in each energy level. Use the notation that lists energy levels by primary quantum number, orbital shell type and total angular momentum, rather than shell number. (Hint – all of the l ...
... a) What orbitals are filled for the protons and neutrons in these two nuclei? List how many neutrons or protons are in each energy level. Use the notation that lists energy levels by primary quantum number, orbital shell type and total angular momentum, rather than shell number. (Hint – all of the l ...
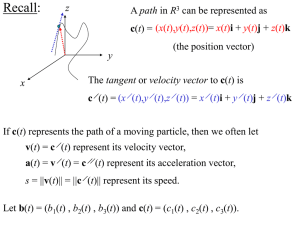
Quantum mechanics is the theory that we use to describe the
... First, a little history. The initial seed that was to later grow into the quantum theory that we have today was Max Planck’s postulate that energy is quantised, or comes in discrete packages, as opposed to existing in an infinitely continuous series of states. He put forward this postulate in order ...
... First, a little history. The initial seed that was to later grow into the quantum theory that we have today was Max Planck’s postulate that energy is quantised, or comes in discrete packages, as opposed to existing in an infinitely continuous series of states. He put forward this postulate in order ...
Chapter 28: Quantum Physics
... The spectrum of hydrogen can only be fully explained if the electron has an intrinsic spin. It is useful to compare this to the Earth spinning on its axis. This cannot be truly what is happening since the surface of the electron would be traveling faster than the speed of light. ...
... The spectrum of hydrogen can only be fully explained if the electron has an intrinsic spin. It is useful to compare this to the Earth spinning on its axis. This cannot be truly what is happening since the surface of the electron would be traveling faster than the speed of light. ...
Postulate 1
... similar idea may have been encountered previously when you considered the dot product of two vectors. If the integral is not equal to one we can normalize the wave function after first evaluating an integral of the form discussed. ...
... similar idea may have been encountered previously when you considered the dot product of two vectors. If the integral is not equal to one we can normalize the wave function after first evaluating an integral of the form discussed. ...
Chapter 37 Early Quantum Theory and Models of the Atom
... occur. This yields the same relation that Bohr had proposed. In addition, it makes more reasonable the fact that the electrons do not radiate, as one would otherwise expect from an accelerating charge. ...
... occur. This yields the same relation that Bohr had proposed. In addition, it makes more reasonable the fact that the electrons do not radiate, as one would otherwise expect from an accelerating charge. ...
CHAPTER 2: Special Theory of Relativity
... is a useful result to relate the total energy of a particle with its momentum. The quantities (E2 – p2c2) and m are invariant quantities. Note that when a particle’s velocity is zero and it has no momentum, “accelerator Equation” correctly gives E0 as the particle’s total energy. There can be mass l ...
... is a useful result to relate the total energy of a particle with its momentum. The quantities (E2 – p2c2) and m are invariant quantities. Note that when a particle’s velocity is zero and it has no momentum, “accelerator Equation” correctly gives E0 as the particle’s total energy. There can be mass l ...
Advanced Quantum Mechanics Syllabus and Introduction
... particles involved. The second respect is much more difficult: Ordinary QM describes particles that have existed for all time and will continue to exist forever. There are a few physical systems that fit this description, at least to a good approximation, but they are not very interesting! Take some ...
... particles involved. The second respect is much more difficult: Ordinary QM describes particles that have existed for all time and will continue to exist forever. There are a few physical systems that fit this description, at least to a good approximation, but they are not very interesting! Take some ...
Simple harmonic motion= motion that repeats itself in an identical
... different directions. Elastic properties allowed the bridge to deform until it was under too much stress and it broke. Is light a matter wave? No you cannot physically touch a light wave. It electric and magnetic fields. When these waves past through the eye, they reflect. When it reaches the retna, ...
... different directions. Elastic properties allowed the bridge to deform until it was under too much stress and it broke. Is light a matter wave? No you cannot physically touch a light wave. It electric and magnetic fields. When these waves past through the eye, they reflect. When it reaches the retna, ...