Atomic Theory Study Guide - Reading Community Schools
... 4. Calculate the energy of absorption and emission spectral lines of the hydrogen atom, and identify the electron transitions that caused the lines. 5. Describe the concept of wave-particle duality, and relate the DeBroglie wavelength of a wave or particle to its momentum. 6. Describe the photoelect ...
... 4. Calculate the energy of absorption and emission spectral lines of the hydrogen atom, and identify the electron transitions that caused the lines. 5. Describe the concept of wave-particle duality, and relate the DeBroglie wavelength of a wave or particle to its momentum. 6. Describe the photoelect ...
Task 1
... 2. According to the usual rules of quantum mechanics, the actual state of the electron may be any superposition of these states. This explains also why the choice of z-axis for the directional quantization of the angular momentum vector is immaterial. ________________________________________________ ...
... 2. According to the usual rules of quantum mechanics, the actual state of the electron may be any superposition of these states. This explains also why the choice of z-axis for the directional quantization of the angular momentum vector is immaterial. ________________________________________________ ...
em waves dual nature atoms and nuclei
... radiating energy” can be explained on the basis of de Broglie hypothesis of wave nature of electron. FIVE MARKS QUESTIONS ...
... radiating energy” can be explained on the basis of de Broglie hypothesis of wave nature of electron. FIVE MARKS QUESTIONS ...
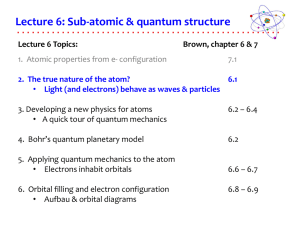
12.1: What are electromagnetic waves?
... EM waves can behave as both a wave and a particle (the particle-wave duality). Phenomenon discovered in 1887 by Heinrich Hertz. Experiment known as the “photo-electric effect”: Shine a light on metal and the metal will eject electrons. Whether it happened depended on the frequency of the lig ...
... EM waves can behave as both a wave and a particle (the particle-wave duality). Phenomenon discovered in 1887 by Heinrich Hertz. Experiment known as the “photo-electric effect”: Shine a light on metal and the metal will eject electrons. Whether it happened depended on the frequency of the lig ...
Quantum Model Worksheet
... PART A – WAVES & QUANTUM MECHANICS 1. What experimental evidence supported de Broglie’s idea that electrons have wave-like properties? ...
... PART A – WAVES & QUANTUM MECHANICS 1. What experimental evidence supported de Broglie’s idea that electrons have wave-like properties? ...
R - McGraw Hill Higher Education
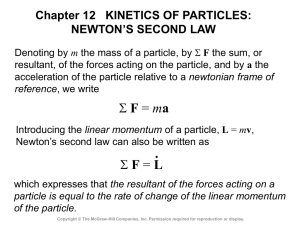
... HO = constant We conclude that the angular momentum of a particle moving under a central force is constant, both in magnitude and direction, and that the particle moves in a plane perpendicular to HO . ...
... HO = constant We conclude that the angular momentum of a particle moving under a central force is constant, both in magnitude and direction, and that the particle moves in a plane perpendicular to HO . ...
Course Description Pre-requests Level Year Number of Study Hours
... 1 – To identify the principles and theories of modern physics and their application areas 2 - To interpretation of physical phenomena involved in decision 3 – To Solving the exercises and questions and exercises covered in the decision ...
... 1 – To identify the principles and theories of modern physics and their application areas 2 - To interpretation of physical phenomena involved in decision 3 – To Solving the exercises and questions and exercises covered in the decision ...
107 chem Assement Q
... c. fundamental state. d. original state. 5. The hydrogen emission spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. the n = 3 state to the n = 2 state. b. the n = 4 state to the n = 2 state. c. the n = 5 state to the n = 2 state. d. the n = 6 ...
... c. fundamental state. d. original state. 5. The hydrogen emission spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. the n = 3 state to the n = 2 state. b. the n = 4 state to the n = 2 state. c. the n = 5 state to the n = 2 state. d. the n = 6 ...
Quantum Theory of the Atom
... lowest energy level (ground state) •When an electron absorbs energy it moves to a higher energy level (excited state) •When an e- drops back down to a lower energy level it gives off a quantum of energy called a “photon” •Only certan atomic spectra are possible and emitted ...
... lowest energy level (ground state) •When an electron absorbs energy it moves to a higher energy level (excited state) •When an e- drops back down to a lower energy level it gives off a quantum of energy called a “photon” •Only certan atomic spectra are possible and emitted ...
Lecture 15 (Slides) September 28
... simply (2nd year). It enables us to calculate allowed energy values, positions of nodes (zero probability of finding a particle) and where a particle is most likely to be found. ...
... simply (2nd year). It enables us to calculate allowed energy values, positions of nodes (zero probability of finding a particle) and where a particle is most likely to be found. ...