1 pt
... What is the name of the term given to the minimum quantity of energy that can be lost or gained by an atom? ...
... What is the name of the term given to the minimum quantity of energy that can be lost or gained by an atom? ...
Physics IV - Exam - Winter 2007/08 Please note:
... • Please WRITE YOUR NAME BELOW. This sheet will be stapled to your answers at the end of the exam. • Please put your name on all of your answer sheets. • Throughout the exam the exam overseers are available to answer your questions, do not hesitate to ask for clarification if needed. ...
... • Please WRITE YOUR NAME BELOW. This sheet will be stapled to your answers at the end of the exam. • Please put your name on all of your answer sheets. • Throughout the exam the exam overseers are available to answer your questions, do not hesitate to ask for clarification if needed. ...
Chapter 7 Name Atomic Structure and Periodicity Any day you don`t
... Conclusions from Einstein and Plank: Energy is quantized. It can occur only in discrete units called ______________. ...
... Conclusions from Einstein and Plank: Energy is quantized. It can occur only in discrete units called ______________. ...
1 Why study Classical Mechanics?
... gone wrong) (see http://savvyparanoia.com/the-fastest-man-made-object-ever-a-nuclear-powered-manholecover-true/ ) which would have been traveling at about 237,500 mph. This still is only vc = 2.2 × 10−4 . These small corrections are important only for extremely fine measurements, where they are easi ...
... gone wrong) (see http://savvyparanoia.com/the-fastest-man-made-object-ever-a-nuclear-powered-manholecover-true/ ) which would have been traveling at about 237,500 mph. This still is only vc = 2.2 × 10−4 . These small corrections are important only for extremely fine measurements, where they are easi ...
Quantum Numbers
... – Number of waves that pass a specific point in a given time – Unit: Hz or waves/sec Recall that Speed = Distance/time (m/sec) ...
... – Number of waves that pass a specific point in a given time – Unit: Hz or waves/sec Recall that Speed = Distance/time (m/sec) ...
Midterm Solution
... tunnel effect, yes is does, potential energy is U, total energy is E and also U + KE, the latter being kinetic energy, so U > E implies negative kinetic energy which does not exist classically, in quantum mechanics this is not a problem at all, the wave function above is for such a scenario U > E, t ...
... tunnel effect, yes is does, potential energy is U, total energy is E and also U + KE, the latter being kinetic energy, so U > E implies negative kinetic energy which does not exist classically, in quantum mechanics this is not a problem at all, the wave function above is for such a scenario U > E, t ...
Chem 150 Problem Set Introductory Quantum Chemistry 1
... a) According to the Bohr model an electron in the ground state of a hydrogen atom orbits the nucleus at a specific radius of 0.53 x 10-10 m. In the quantum mechanical description of the hydrogen atom, the most probable distance of the electron from the nucleus is 0.53 x 10 -10 m. Why are these two s ...
... a) According to the Bohr model an electron in the ground state of a hydrogen atom orbits the nucleus at a specific radius of 0.53 x 10-10 m. In the quantum mechanical description of the hydrogen atom, the most probable distance of the electron from the nucleus is 0.53 x 10 -10 m. Why are these two s ...
Scotty may soon be able to beam us up
... the microscopic world, quantum theorists have found that they simply cannot pin down the smallest bits of atoms at all. Some are so tiny that they are smaller than the smallest rays of light that we need to look at them, so that in observing them we are liable to "bump into" them and change them. Mo ...
... the microscopic world, quantum theorists have found that they simply cannot pin down the smallest bits of atoms at all. Some are so tiny that they are smaller than the smallest rays of light that we need to look at them, so that in observing them we are liable to "bump into" them and change them. Mo ...
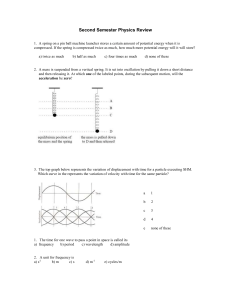
Second Semester Physics Review
... 3. The top graph below represents the variation of displacement with time for a particle executing SHM. Which curve in the represents the variation of velocity with time for the same particle? ...
... 3. The top graph below represents the variation of displacement with time for a particle executing SHM. Which curve in the represents the variation of velocity with time for the same particle? ...
Chapter 7
... The product of the wavelength and the frequency, though, is a constant. c = l n, where c is the speed of light. Thus, if we know the frequency, we can find the wavelength and vice versa. LEP #1(a). ...
... The product of the wavelength and the frequency, though, is a constant. c = l n, where c is the speed of light. Thus, if we know the frequency, we can find the wavelength and vice versa. LEP #1(a). ...
Ideas of Modern Physics
... 8. A hydrogen atom has quantum states with energies -13.6eV/n2. Which of the following transitions emits the shortest wavelength photon? a. n=2 to n=1 b. n=3 to n=2 c. n=3 to n=1 d. n=4 to n=3 e. all emit the same wavelength photon ...
... 8. A hydrogen atom has quantum states with energies -13.6eV/n2. Which of the following transitions emits the shortest wavelength photon? a. n=2 to n=1 b. n=3 to n=2 c. n=3 to n=1 d. n=4 to n=3 e. all emit the same wavelength photon ...
vocab chap 6
... packed nucleus and that atoms are mostly empty space; also discovered the proton ...
... packed nucleus and that atoms are mostly empty space; also discovered the proton ...
quantumwaves
... •Two de Broglie relations •They have all the properties (and equations) that described waves before, together with some new ones •Rewrite the de Broglie relations in terms of k and : ...
... •Two de Broglie relations •They have all the properties (and equations) that described waves before, together with some new ones •Rewrite the de Broglie relations in terms of k and : ...
Second Semester Final Practice
... 20. The property of sound called intensity is: a) proportional to the rate at which sound energy flows through an area normal to the direction of wave propagation. b) inversely proportional to the rate at which sound energy flows through an area normal to the direction of propagation. c) proportiona ...
... 20. The property of sound called intensity is: a) proportional to the rate at which sound energy flows through an area normal to the direction of wave propagation. b) inversely proportional to the rate at which sound energy flows through an area normal to the direction of propagation. c) proportiona ...
Chapter 7 Worksheet November 1
... 17 (BONUS) You are at a baseball game and a person sitting next to you is trying to impress their date by telling them that the baseball has vibrations, and that its position in space cannot totally be determined by the batter. You know that such concepts only really apply to infinitesimally small p ...
... 17 (BONUS) You are at a baseball game and a person sitting next to you is trying to impress their date by telling them that the baseball has vibrations, and that its position in space cannot totally be determined by the batter. You know that such concepts only really apply to infinitesimally small p ...
Arrangement of Electrons in Atoms I. The Development of a New
... Before the 1900’s, scientists thought light behaved only as waves. This belief changed when it was discovered that light also has particle-like characteristics. This is known as the wave-particle duality of light. ...
... Before the 1900’s, scientists thought light behaved only as waves. This belief changed when it was discovered that light also has particle-like characteristics. This is known as the wave-particle duality of light. ...