Chemical Formula Analysis
... Definition: compounds that contain water molecules trapped in their crystal lattice structure Ex) MgSO4 7 H2O (Epsom salts) - dot represents the physical separation of water molecules from the ionic compound in which they are trapped ...
... Definition: compounds that contain water molecules trapped in their crystal lattice structure Ex) MgSO4 7 H2O (Epsom salts) - dot represents the physical separation of water molecules from the ionic compound in which they are trapped ...
Molecular Formulas - Brookwood High School
... 1. Analysis of a chemical used in photographic developing fluid indicates a chemical composition of 65.45% C, 5.45% H, and 29.09% O. The molar mass is found to be 110 g/mol. Determine the molecular formula. ...
... 1. Analysis of a chemical used in photographic developing fluid indicates a chemical composition of 65.45% C, 5.45% H, and 29.09% O. The molar mass is found to be 110 g/mol. Determine the molecular formula. ...
Rigid and non-rigid Rotors
... The diatomic molecule comprises two atoms, numbered 1 and 2, with respective atomic masses m1 and m2, a distance r apart. The center of mass (which is at a distance r1 from atom 1 and r2 from atom 2, such that r1+r2 = r) is located by recognizing that the moments of the masses should be equal around ...
... The diatomic molecule comprises two atoms, numbered 1 and 2, with respective atomic masses m1 and m2, a distance r apart. The center of mass (which is at a distance r1 from atom 1 and r2 from atom 2, such that r1+r2 = r) is located by recognizing that the moments of the masses should be equal around ...
A Binary Star as a Quantum System
... æ nx p x ö æ nyp y ö æ nz p z ö y (x, y, z) = ç ÷ sin ç ÷ sin ç ...
... æ nx p x ö æ nyp y ö æ nz p z ö y (x, y, z) = ç ÷ sin ç ÷ sin ç ...
Rigid Rotations
... Now, we could proceed to work out the relationships for ⎡⎣ L̂ y , L̂z ⎤⎦ and ⎡⎣ L̂z , L̂ x ⎤⎦ . However, we note that our answer must always be invariant to a cyclic permutation x → y, y → z, z → x . This must be the case because our labeling of the x, y and z axes is totally arbitrary: if we chose ...
... Now, we could proceed to work out the relationships for ⎡⎣ L̂ y , L̂z ⎤⎦ and ⎡⎣ L̂z , L̂ x ⎤⎦ . However, we note that our answer must always be invariant to a cyclic permutation x → y, y → z, z → x . This must be the case because our labeling of the x, y and z axes is totally arbitrary: if we chose ...
formula mass.
... The Avogadro number was named in honor of Amedeo Avogadro who discovered that a mole of any gas under the same conditions has the same number of molecules. Johann Josef Loschmidt, a German physicist, named and discovered the Avogadro number. Loschmidt realized that a mole of any substance—be i ...
... The Avogadro number was named in honor of Amedeo Avogadro who discovered that a mole of any gas under the same conditions has the same number of molecules. Johann Josef Loschmidt, a German physicist, named and discovered the Avogadro number. Loschmidt realized that a mole of any substance—be i ...
Section 2.6
... Empirical Formula • Indicate the actual • Only give the numbers and types of relative number of atoms in a molecule. atoms of each type in a molecule. ...
... Empirical Formula • Indicate the actual • Only give the numbers and types of relative number of atoms in a molecule. atoms of each type in a molecule. ...
Exam I CHEM 1303.001 KEY FALL 2009 Part 1. Nomenclature. 10
... Part 2. Multiple choice (use answer card). 90 pts. total. 3 pts. each. Indicate the BEST answer for the questions in this section by completely filling in the appropriate space on the answer card with a #2 pencil. Mark only one space per question. How many of the following numbers have 4 significant ...
... Part 2. Multiple choice (use answer card). 90 pts. total. 3 pts. each. Indicate the BEST answer for the questions in this section by completely filling in the appropriate space on the answer card with a #2 pencil. Mark only one space per question. How many of the following numbers have 4 significant ...
A Quantum Mechanical Model for Vibration and Rotation of Molecules
... Our interest is to model the oscillatory motion of the diatomic molecule (relative motions of atoms). Also – of interest kinetic and potential energy of the diatomic molecule during the oscillatory motion. Not necessarily the energies of individual atoms of the ...
... Our interest is to model the oscillatory motion of the diatomic molecule (relative motions of atoms). Also – of interest kinetic and potential energy of the diatomic molecule during the oscillatory motion. Not necessarily the energies of individual atoms of the ...
Honors Chemistry Chapter 10 Student Notes
... Mole-Mass and Mole-Volume Relationships For starters in chemistry, we have to be able to convert between moles, grams, and molecules/atoms of substance (also liters when we work with gases). The “mole map”: ...
... Mole-Mass and Mole-Volume Relationships For starters in chemistry, we have to be able to convert between moles, grams, and molecules/atoms of substance (also liters when we work with gases). The “mole map”: ...
Prelab: Empirical Formulas
... Once the masses of Mg and Cl in the compound formed are determined experimentally, the procedures given above for determining the empirical formula will be applied. Safety Note: We will be evaporating excess HCl in this experiment and so this must be done in the HOOD! The effect of certain experimen ...
... Once the masses of Mg and Cl in the compound formed are determined experimentally, the procedures given above for determining the empirical formula will be applied. Safety Note: We will be evaporating excess HCl in this experiment and so this must be done in the HOOD! The effect of certain experimen ...
NAME REVIEW 1: JUST THE BASICS ___1) In which material are
... 20) 1) HI it is produced endothermically and that means more energy is absorbed by the breaking of bonds than is released as the new H-I polar covalent bond(s) is (are) produced. Thus HI is less stable than the reactants. 21) 3 an increase in temp favors the endo. rxn which in this case is the forwa ...
... 20) 1) HI it is produced endothermically and that means more energy is absorbed by the breaking of bonds than is released as the new H-I polar covalent bond(s) is (are) produced. Thus HI is less stable than the reactants. 21) 3 an increase in temp favors the endo. rxn which in this case is the forwa ...
Midterm Review File
... 19. Answer the following questions about the periodic table. a. Explain why noble gases are inert and do not form ions. b. Identify the name of the group that contains the element fluorine _______________ c. Give the name of the element in the alkali group that has the greatest electron affinity ___ ...
... 19. Answer the following questions about the periodic table. a. Explain why noble gases are inert and do not form ions. b. Identify the name of the group that contains the element fluorine _______________ c. Give the name of the element in the alkali group that has the greatest electron affinity ___ ...
Chapter-09_Summary
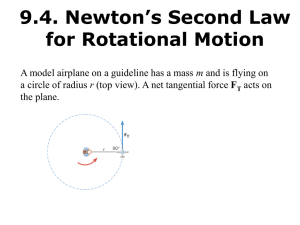
... Angular momentum of a rigid body rotating with an angular momentum about a fixed axis and having a momentum of inertia I (where angular momentum is L) is: Principle of conservation of angular momentum states that the total angular momentum of a system remains constant as long as the net average exte ...
... Angular momentum of a rigid body rotating with an angular momentum about a fixed axis and having a momentum of inertia I (where angular momentum is L) is: Principle of conservation of angular momentum states that the total angular momentum of a system remains constant as long as the net average exte ...
Chemistry 330
... to vibrate. The symmetric and antisymmetric stretches are independent, and one can be excited without affecting the other: they are normal modes. The two perpendicular bending motions are also normal modes. ...
... to vibrate. The symmetric and antisymmetric stretches are independent, and one can be excited without affecting the other: they are normal modes. The two perpendicular bending motions are also normal modes. ...
Accelerated Chemistry
... the gram formula mass of the empirical formula and its relationship to the gram formula mass of the molecular formula to find what number to multiply the empirical formula by to find the molecular formula (sounds more complicated than it is) example 5 - Find the molecular formula of a compound (mw = ...
... the gram formula mass of the empirical formula and its relationship to the gram formula mass of the molecular formula to find what number to multiply the empirical formula by to find the molecular formula (sounds more complicated than it is) example 5 - Find the molecular formula of a compound (mw = ...