PHYSICS 51: Introduction
... For spherical shapes all the mass acts as if its at the center of the sphere (when outside the sphere) For multiple masses add the gravitational forces as vectors ...
... For spherical shapes all the mass acts as if its at the center of the sphere (when outside the sphere) For multiple masses add the gravitational forces as vectors ...
Fractional Quantum Hall States with Non
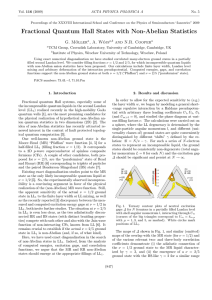
... the layer width w, we began by modeling a general short-range repulsive interaction by a Haldane pseudopotential with arbitrary three leading coefficients U1 , U3 , U5 (and Um>5 = 0), and studied the phase diagram at various filling factors ν. The calculations were carried out on a sphere, where the ...
... the layer width w, we began by modeling a general short-range repulsive interaction by a Haldane pseudopotential with arbitrary three leading coefficients U1 , U3 , U5 (and Um>5 = 0), and studied the phase diagram at various filling factors ν. The calculations were carried out on a sphere, where the ...
Modern Physics 2-Quantum Optics
... • Planck hypothesized that charged particles could radiate energy only in discrete portions called quanta. • He proposed that each microscopic oscillating charged particle had some kind of fundamental portion of energy, which was proportional to the frequency of its oscillation. • According to Planc ...
... • Planck hypothesized that charged particles could radiate energy only in discrete portions called quanta. • He proposed that each microscopic oscillating charged particle had some kind of fundamental portion of energy, which was proportional to the frequency of its oscillation. • According to Planc ...
Phys 197 Homework Solution 41A Q3.
... Neglect the 1/ℓ2 to get the fractional change as 2/ℓ = 4.6 × 10−48 . This means that the next allowed orbit is farther out by a distance of (4.6 × 10−48 )(1.5 × 109 m) = 7 × 10−39 m. Q4. Why is the analysis of the helium atom much more complex than that of the hydrogen atom, either in a Bohr type of ...
... Neglect the 1/ℓ2 to get the fractional change as 2/ℓ = 4.6 × 10−48 . This means that the next allowed orbit is farther out by a distance of (4.6 × 10−48 )(1.5 × 109 m) = 7 × 10−39 m. Q4. Why is the analysis of the helium atom much more complex than that of the hydrogen atom, either in a Bohr type of ...
6 - The Quantum Atomic Model SCH4U – Structure and Properties of
... Bohr saw atomic spectra as evidence that the energy of electrons in an atom is “___________________” (limited to only certain __________________________) Bohr introduced the first “_________________ model” the atom by concluding that electrons in an atom are confided to discrete “___________________ ...
... Bohr saw atomic spectra as evidence that the energy of electrons in an atom is “___________________” (limited to only certain __________________________) Bohr introduced the first “_________________ model” the atom by concluding that electrons in an atom are confided to discrete “___________________ ...
Quantum Reality
... If one boson is in a particular quantum state, all other bosons are "invited in" to share the same state. The more bosons that pile into the state, the stronger becomes the tendency for others to join them. In such a state, a very large number of particle will have a single quantum ...
... If one boson is in a particular quantum state, all other bosons are "invited in" to share the same state. The more bosons that pile into the state, the stronger becomes the tendency for others to join them. In such a state, a very large number of particle will have a single quantum ...
Chapter 7 - ETSU.edu
... electron jump between orbits must be accompanied by an emitted or absorbed amount of electromagnetic energy hν. The orbits that the electrons travel in are shown as grey circles; their radius increases n2, where n is the principal quantum number. The 3→2 transition depicted here produces the first l ...
... electron jump between orbits must be accompanied by an emitted or absorbed amount of electromagnetic energy hν. The orbits that the electrons travel in are shown as grey circles; their radius increases n2, where n is the principal quantum number. The 3→2 transition depicted here produces the first l ...
Energy: A Physicist`s View - University of Colorado Boulder
... electromagnetic fields existing in an electromagnetic environment." "Based on Einstein's theories of quantum physics, these energetic concepts are being integrated into medicine for a comprehensive approach to disease diagnosis, prevention, and treatment.” Joan Stafantos ...
... electromagnetic fields existing in an electromagnetic environment." "Based on Einstein's theories of quantum physics, these energetic concepts are being integrated into medicine for a comprehensive approach to disease diagnosis, prevention, and treatment.” Joan Stafantos ...
Quantum
... releasing a large amount of concentrated energy in the process. Experimentally this is done with the addition of a neutron. The atom then splits into two atoms releasing high energy gamma rays that can be harnessed in nuclear reactors. 2. Nuclear fusion: The inverse of what occurs in fission. The at ...
... releasing a large amount of concentrated energy in the process. Experimentally this is done with the addition of a neutron. The atom then splits into two atoms releasing high energy gamma rays that can be harnessed in nuclear reactors. 2. Nuclear fusion: The inverse of what occurs in fission. The at ...
some aspects of strange matter : stars and strangelets
... The quantum nature of light was used by Planck in 1900 to describe the character of black body radiation. In 1905 Einstein confirmed the quantum nature by explaining the photo-electric effect. Later in 1923 scattering of X-rays and γrays on electrons in atoms was explained again with the quantum n ...
... The quantum nature of light was used by Planck in 1900 to describe the character of black body radiation. In 1905 Einstein confirmed the quantum nature by explaining the photo-electric effect. Later in 1923 scattering of X-rays and γrays on electrons in atoms was explained again with the quantum n ...
Course Code: Title of the Course
... This course is spread over three modules (Force and Linear Motion, Energy and Angular Motion). It utilizes mathematics and physics to investigate the laws and concepts of mechanics. It begins with measurement systems and Newtonian concepts of force and motion. Motion is examined in two and three dim ...
... This course is spread over three modules (Force and Linear Motion, Energy and Angular Motion). It utilizes mathematics and physics to investigate the laws and concepts of mechanics. It begins with measurement systems and Newtonian concepts of force and motion. Motion is examined in two and three dim ...
Sizes in the Universe - Indico
... The anthropic argument: “Constants of Nature are what they are (very big or very small) because if they weren’t, we wouldn’t be here to observe them …” ...
... The anthropic argument: “Constants of Nature are what they are (very big or very small) because if they weren’t, we wouldn’t be here to observe them …” ...
Lecture 3: Heterostructures, Quasielectric Fields, and Quantum
... experience this field and be accelerated in the positive x-direction. Note that the quasi electric field for the valence band will in general have a different magnitude and can have a different sign. In this case, the valence band quasi electric field has a value EV∗ = +1.32 × 104 V/cm. [For materia ...
... experience this field and be accelerated in the positive x-direction. Note that the quasi electric field for the valence band will in general have a different magnitude and can have a different sign. In this case, the valence band quasi electric field has a value EV∗ = +1.32 × 104 V/cm. [For materia ...