Newton`s Cradle - Mercer Physics
... such that v1i is the initial velocity of m 1 , v1f is the final velocity of m 1 , and v2f is the final velocity of m 2 . This equation states that the net linear momentum of the system is conserved. Furthermore, an equation stating conservation of kinetic energy for elastic collisions can be written ...
... such that v1i is the initial velocity of m 1 , v1f is the final velocity of m 1 , and v2f is the final velocity of m 2 . This equation states that the net linear momentum of the system is conserved. Furthermore, an equation stating conservation of kinetic energy for elastic collisions can be written ...
A r - Stony Brook University
... makes a transition from the quantum state ni to the quantum state nf ...
... makes a transition from the quantum state ni to the quantum state nf ...
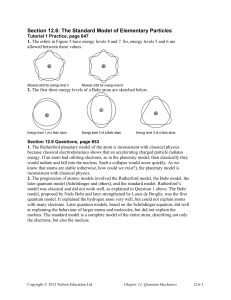
Section 12.6: The Standard Model of Elementary Particles
... would radiate and fall into the nucleus. Such a collapse would occur quickly. As we know that atoms are stable (otherwise, how could we exist?), the planetary model is inconsistent with classical physics. 2. The progression of atomic models involved the Rutherford model, the Bohr model, the later qu ...
... would radiate and fall into the nucleus. Such a collapse would occur quickly. As we know that atoms are stable (otherwise, how could we exist?), the planetary model is inconsistent with classical physics. 2. The progression of atomic models involved the Rutherford model, the Bohr model, the later qu ...
Quantum Mechanics review WS
... impossible to know both the exact momentum and location of a particle simultaneously. The better you know one quantity, the more uncertain you must be of the other. 22. According to quantum mechanics theory, is it possible to track the motion of a particle from start to end? What does the theory say ...
... impossible to know both the exact momentum and location of a particle simultaneously. The better you know one quantity, the more uncertain you must be of the other. 22. According to quantum mechanics theory, is it possible to track the motion of a particle from start to end? What does the theory say ...
Equations of Motion Computational Physics Orbital Motion
... Radial Force dependent on position only: Angular Momentum conserved; Motion in a plane. ...
... Radial Force dependent on position only: Angular Momentum conserved; Motion in a plane. ...
Physics Qualifier Part I—Spring 2010 7-Minute Questions α
... b. Linearly polarized light of variable frequency ω is shone on the electron. The potential energy of the electron in the light field is Vf (x,y,t) = Vox cos (ωt). What are the selection rules for transitions between the four lowest energy levels already found? 9. For a particle of mass m in an infi ...
... b. Linearly polarized light of variable frequency ω is shone on the electron. The potential energy of the electron in the light field is Vf (x,y,t) = Vox cos (ωt). What are the selection rules for transitions between the four lowest energy levels already found? 9. For a particle of mass m in an infi ...
QM L-6
... terms of probabilities and not specific numbers. Therefore, instead of finding the average value of any term (for example position of particle x ), we find the expectation value of that.
Ni xi
...
... terms of probabilities and not specific numbers. Therefore, instead of finding the average value of any term (for example position of particle x ), we find the expectation value
January 1998
... where a is a positive constant. The other terms in the hydrogen atom Hamiltonian do not lift the degeneracy of the n = 1 level and may be ignored in this problem. a) ...
... where a is a positive constant. The other terms in the hydrogen atom Hamiltonian do not lift the degeneracy of the n = 1 level and may be ignored in this problem. a) ...
PHY492: Nuclear & Particle Physics Lecture 5 Angular momentum Nucleon magnetic moments
... Five terms (+ means weaker binding) in a prediction of the B.E. – r ~A1/3, Binding is short ranged, depending only on nearest neighbors. This leads to a B.E. term proportional to A: –a1 A. – The surface nucleons are not surrounded by others. This leads to a term proportional to A2/3 that weakens the ...
... Five terms (+ means weaker binding) in a prediction of the B.E. – r ~A1/3, Binding is short ranged, depending only on nearest neighbors. This leads to a B.E. term proportional to A: –a1 A. – The surface nucleons are not surrounded by others. This leads to a term proportional to A2/3 that weakens the ...
Chapter 4 - Arrangement of Electrons in Atoms
... a. Electrons are emitted from a metal when light shines on the metal 2. Radiant energy is transferred in units (or quanta) of energy called photons a. A photon is a particle of energy having a rest mass of zero and carrying a quantum of energy b. A quantum is the minimum amount of energy that can be ...
... a. Electrons are emitted from a metal when light shines on the metal 2. Radiant energy is transferred in units (or quanta) of energy called photons a. A photon is a particle of energy having a rest mass of zero and carrying a quantum of energy b. A quantum is the minimum amount of energy that can be ...
T3 F2013 9 30
... b. Write down three equations, two by balancing the forces, and one by balancing the torque. c. In unit-vector notation, what is the force on the beam from the hinge? ...
... b. Write down three equations, two by balancing the forces, and one by balancing the torque. c. In unit-vector notation, what is the force on the beam from the hinge? ...
A Helium atom has a nuclear charge of Ze, where Z=2. One of the
... A Helium atom has a nuclear charge of Ze, where Z=2. One of the electrons is removed leaving an atom that resembles a Hydrogen atom but with twice the nuclear charge. What are the energy levels in this atom? a) En= - mZe4 / ( e028n2h2) b) En= - mZ2e4 / ( e028n2h2) c) En= - mZ4e4 / ( e028n2h2) d) En= ...
... A Helium atom has a nuclear charge of Ze, where Z=2. One of the electrons is removed leaving an atom that resembles a Hydrogen atom but with twice the nuclear charge. What are the energy levels in this atom? a) En= - mZe4 / ( e028n2h2) b) En= - mZ2e4 / ( e028n2h2) c) En= - mZ4e4 / ( e028n2h2) d) En= ...