ch11 - alcohols and ethers
... 1. Ethers by Intermolecular Dehydration of Alcohol l Primary alcohols can dehydrate to ethers è This reaction occurs at lower temperature than the competing dehydration to an alkene è This method generally does not work with secondary or tertiary ...
... 1. Ethers by Intermolecular Dehydration of Alcohol l Primary alcohols can dehydrate to ethers è This reaction occurs at lower temperature than the competing dehydration to an alkene è This method generally does not work with secondary or tertiary ...
Note Sheets and Sample Problems
... o e is charge on electron in Coulombs, (C) and m is its mass. o Thomson discovered that he could repeat this deflection and calculation using electrodes of different metals ∴ all metals contained electrons and ALL ATOMS contained electrons o Furthermore, all atoms were neutral ∴ there must be some ( ...
... o e is charge on electron in Coulombs, (C) and m is its mass. o Thomson discovered that he could repeat this deflection and calculation using electrodes of different metals ∴ all metals contained electrons and ALL ATOMS contained electrons o Furthermore, all atoms were neutral ∴ there must be some ( ...
Addition of H 2 O to an Alkene
... between the reactants and the transition state. – Ea determines the rate of reaction. – If Ea is large very few molecular collisions occur with sufficient energy to reach the transition state, and the reaction is slow. – If Ea is small many collisions generate sufficient energy to reach the transiti ...
... between the reactants and the transition state. – Ea determines the rate of reaction. – If Ea is large very few molecular collisions occur with sufficient energy to reach the transition state, and the reaction is slow. – If Ea is small many collisions generate sufficient energy to reach the transiti ...
Alcohol - djkuranui
... • Each alcohol is defined by having a C-OH bond. So there are three other bonds on that carbon… to determine what type of alcohol it is, simple count the things bonded to the carbon that aren’t hydrogens. (often labeled as “R” groups) • 3 Types – Primary Alcohol • Alcohol with only 1 “R” group…. – S ...
... • Each alcohol is defined by having a C-OH bond. So there are three other bonds on that carbon… to determine what type of alcohol it is, simple count the things bonded to the carbon that aren’t hydrogens. (often labeled as “R” groups) • 3 Types – Primary Alcohol • Alcohol with only 1 “R” group…. – S ...
10 | Carbon: More Than Just Another Element
... octet configuration. In contrast, the elements boron and nitrogen form three bonds in molecular compounds; oxygen forms two bonds; and hydrogen and the halogens form one bond. With a larger number of bonds comes the opportunity to create more complex structures. This will become increasingly evident ...
... octet configuration. In contrast, the elements boron and nitrogen form three bonds in molecular compounds; oxygen forms two bonds; and hydrogen and the halogens form one bond. With a larger number of bonds comes the opportunity to create more complex structures. This will become increasingly evident ...
Review Unit: Chemistry Review
... possible. Science would not progress very far without the increasingly advanced technologies available to scientists. Often scientific advances have to wait on the development of technologies for research to be done; for example, glassware, the battery, the laser, and the computer. Often science is ...
... possible. Science would not progress very far without the increasingly advanced technologies available to scientists. Often scientific advances have to wait on the development of technologies for research to be done; for example, glassware, the battery, the laser, and the computer. Often science is ...
chemical equilibrium
... • you get to the equilibrium position quicker but with a reduced yield because the increased temperature moves the equilibrium to the left • In many industrial processes a compromise temperature is used To reduce the problem one must look for a way of increasing the rate of a reaction without decrea ...
... • you get to the equilibrium position quicker but with a reduced yield because the increased temperature moves the equilibrium to the left • In many industrial processes a compromise temperature is used To reduce the problem one must look for a way of increasing the rate of a reaction without decrea ...
Electrophilic Aromatic Substitution and Substituted Benzenes
... • Seven resonance structures can be drawn for benzaldehyde (C6H5CHO). Because three of them place a positive charge on a carbon atom of the benzene ring, the CHO group withdraws electrons from the benzene ring by a resonance effect. ...
... • Seven resonance structures can be drawn for benzaldehyde (C6H5CHO). Because three of them place a positive charge on a carbon atom of the benzene ring, the CHO group withdraws electrons from the benzene ring by a resonance effect. ...
Dynamic Multi-Component Covalent Assembly for the Binding of
... combinatorial libraries, and sensors 1 , 2 , 3 , 4 , 5 , 6 , 7 . Although the addition of amines to carbonyls to create imines8,9,10,11 or hemiaminals12,13, and the association of boronic acids with diols to form cyclic boronate esters14,15, have been widely explored as reversible systems, the use o ...
... combinatorial libraries, and sensors 1 , 2 , 3 , 4 , 5 , 6 , 7 . Although the addition of amines to carbonyls to create imines8,9,10,11 or hemiaminals12,13, and the association of boronic acids with diols to form cyclic boronate esters14,15, have been widely explored as reversible systems, the use o ...
Document
... • Seven resonance structures can be drawn for benzaldehyde (C6H5CHO). Because three of them place a positive charge on a carbon atom of the benzene ring, the CHO group withdraws electrons from the benzene ring by a resonance effect. ...
... • Seven resonance structures can be drawn for benzaldehyde (C6H5CHO). Because three of them place a positive charge on a carbon atom of the benzene ring, the CHO group withdraws electrons from the benzene ring by a resonance effect. ...
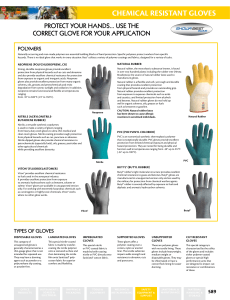
chemical reSiStant GloveS
... Natural rubber, the most elastic substance known, is found in over two hundred plants including the rubber tree (Hevea Brasiliensis), the source of natural rubber latex used to manufacture gloves. Natural rubber is a flexible and soft, yet tough and durable coating that provides excellent protection ...
... Natural rubber, the most elastic substance known, is found in over two hundred plants including the rubber tree (Hevea Brasiliensis), the source of natural rubber latex used to manufacture gloves. Natural rubber is a flexible and soft, yet tough and durable coating that provides excellent protection ...
Common Student Misconceptions
... Bases are substances that accept or react with the H+ ions formed by acids. Hydroxide ions, OH–, react with the H+ ions to form water: H+(aq) + OH–(aq) Æ H2O(l) Common bases are NH3 (ammonia), Draino, milk of magnesia. Compounds that do not contain OH– ions can also be bases. • Proton transfer betwe ...
... Bases are substances that accept or react with the H+ ions formed by acids. Hydroxide ions, OH–, react with the H+ ions to form water: H+(aq) + OH–(aq) Æ H2O(l) Common bases are NH3 (ammonia), Draino, milk of magnesia. Compounds that do not contain OH– ions can also be bases. • Proton transfer betwe ...
Microsoft Word - Open Access Repository of Indian Theses
... Summary The thesis entitled "Photochemistry and chemistry of organic compounds in solution and in zeolitic environment" has been divided in to three parts. Part I contains chapter I and which describes the introduction of the zeolites and photochemical reactions in zeolites. Part II is divided into ...
... Summary The thesis entitled "Photochemistry and chemistry of organic compounds in solution and in zeolitic environment" has been divided in to three parts. Part I contains chapter I and which describes the introduction of the zeolites and photochemical reactions in zeolites. Part II is divided into ...
Chapter 9
... • Alcohols, ethers, and epoxides exhibit dipole-dipole interactions because they have a bent structure with two polar bonds. • Alcohols are capable of intermolecular hydrogen bonding. Thus, alcohols are more polar than ethers and epoxides. ...
... • Alcohols, ethers, and epoxides exhibit dipole-dipole interactions because they have a bent structure with two polar bonds. • Alcohols are capable of intermolecular hydrogen bonding. Thus, alcohols are more polar than ethers and epoxides. ...
5.2 Calculations of Enthalpy Changes (SL/HL)
... Energy is required to break bonds. Energy is released when bonds form. In an exothermic reaction, the amount of energy required to break the bonds of the reactants is less then the amount of energy released when the bonds form in the products. Enthalpy ...
... Energy is required to break bonds. Energy is released when bonds form. In an exothermic reaction, the amount of energy required to break the bonds of the reactants is less then the amount of energy released when the bonds form in the products. Enthalpy ...
Chemistry Worksheets
... Fill in the blanks in the following worksheet. Please keep in mind that the isotope represented by each space may NOT be the most common isotope or the one closest in atomic mass to the value on the periodic table. Atomic symbol ...
... Fill in the blanks in the following worksheet. Please keep in mind that the isotope represented by each space may NOT be the most common isotope or the one closest in atomic mass to the value on the periodic table. Atomic symbol ...
- sartep.com
... 68._______________Which of the molecules has four sp3 hybrid bonds? 69._______________Which substance would have the greatest melting point? 70._______________In which of the choices is there polar double bonding in a non-polar molecule? 71. ________________. . .C10H12O4S(s) + . . O2(g) . . . CO2( ...
... 68._______________Which of the molecules has four sp3 hybrid bonds? 69._______________Which substance would have the greatest melting point? 70._______________In which of the choices is there polar double bonding in a non-polar molecule? 71. ________________. . .C10H12O4S(s) + . . O2(g) . . . CO2( ...
File - cpprashanths Chemistry
... which may then explode . It is for this reason only ethers are purified by mixing with ferrous sulphate solution which reduces the hydrogen peroxide to non explosive alcohols . ...
... which may then explode . It is for this reason only ethers are purified by mixing with ferrous sulphate solution which reduces the hydrogen peroxide to non explosive alcohols . ...
Chapter 22 and 23 Study Guide
... You do not have to do this assignment. You will not receive points for doing it. You should practice these questions until you can do them WITHOUT your book, notes or help from anyone. You should review homework, quizzes and other assignments for complete test preparation. These questions ar ...
... You do not have to do this assignment. You will not receive points for doing it. You should practice these questions until you can do them WITHOUT your book, notes or help from anyone. You should review homework, quizzes and other assignments for complete test preparation. These questions ar ...