Chapter 12
... The structures of borate minerals are complex and diverse, but they charac teristically contain trigonal B0 3 or tetrahedral B0 4 units in large boron-oxygen anions. Some oxygen atoms in borate minerals are monoprotonated to give hy droxyl groups, while others are diprotonated to give waters of hy ...
... The structures of borate minerals are complex and diverse, but they charac teristically contain trigonal B0 3 or tetrahedral B0 4 units in large boron-oxygen anions. Some oxygen atoms in borate minerals are monoprotonated to give hy droxyl groups, while others are diprotonated to give waters of hy ...
A convenient method for the preparation of oxazaborolidine catalyst
... The oxazaborolidine (CBS reagent)-catalyzed asymmetric reduction methodology has been extensively used for this purpose.2–4 Even though the parent catalyst H-CBS 4, prepared in situ using a,a-diphenylpyrrolidinemethanol and BH3–THF, has been found to give good results in asymmetric reductions, the c ...
... The oxazaborolidine (CBS reagent)-catalyzed asymmetric reduction methodology has been extensively used for this purpose.2–4 Even though the parent catalyst H-CBS 4, prepared in situ using a,a-diphenylpyrrolidinemethanol and BH3–THF, has been found to give good results in asymmetric reductions, the c ...
Chapter 4 Stereochemistry and Chirality Flow chart for determining
... Enantiotopic: If you have a methylene group of the form X- CH2 -Z, and you replace one of the hydrogens of the CH2 with a dummy atom and then you independently replace the other hydrogen of the CH 2 group with a dummy atom (Y), the two molecules thus created will be enantiomers of each other. The pr ...
... Enantiotopic: If you have a methylene group of the form X- CH2 -Z, and you replace one of the hydrogens of the CH2 with a dummy atom and then you independently replace the other hydrogen of the CH 2 group with a dummy atom (Y), the two molecules thus created will be enantiomers of each other. The pr ...
Novel Synthesis of Schiff bases Bearing Glucosamine Moiety
... with salicylaldehyde have been shown to give stable complexes with transition-metal ions such as Cu (II), Fe (III), and Co (II) and can be applied as asymmetric catalysts14. On the other hand Pérez et al. have reported the first detailed study on the Schiff bases formed by reaction of D-glucosamine ...
... with salicylaldehyde have been shown to give stable complexes with transition-metal ions such as Cu (II), Fe (III), and Co (II) and can be applied as asymmetric catalysts14. On the other hand Pérez et al. have reported the first detailed study on the Schiff bases formed by reaction of D-glucosamine ...
chemical change
... Acetic acid is known to contain the elements carbon, hydrogen and oxygen only. A 4.24 g sample of acetic acid is combusted to form 6.21 g of carbon dioxide, and 2.54 g of water. Calculate the percentage composition of acetic acid, and use this to determine the empirical formula. ...
... Acetic acid is known to contain the elements carbon, hydrogen and oxygen only. A 4.24 g sample of acetic acid is combusted to form 6.21 g of carbon dioxide, and 2.54 g of water. Calculate the percentage composition of acetic acid, and use this to determine the empirical formula. ...
Chemdraw B&W - Chemistry Courses
... except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
... except in very strong acid • The C=O group is strongly electron-withdrawing, making the N a very weak base • Addition of a proton occurs on O but this destroys the double bond character of C=O as a requirement of stabilization by N ...
No Slide Title
... somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a partial negative charge on the oxygen atom (-) and partial positive charges on the hydrogen atoms (+), where “” indicates a small positive ...
... somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a partial negative charge on the oxygen atom (-) and partial positive charges on the hydrogen atoms (+), where “” indicates a small positive ...
Activation of Nitrous Oxide and Selective Epoxidation of Alkenes
... species was obtained,20 which was also identical to the spectrum of authentic K12[MnII2(H2O)2ZnW(ZnW9O34)2].21 No other peaks, for example, at g ) ∼4, attributable to a Mn(IV) POM were observed. The Mn(II) concentration was significant and was estimated to be at least 70 ( 10% of the total Mn by com ...
... species was obtained,20 which was also identical to the spectrum of authentic K12[MnII2(H2O)2ZnW(ZnW9O34)2].21 No other peaks, for example, at g ) ∼4, attributable to a Mn(IV) POM were observed. The Mn(II) concentration was significant and was estimated to be at least 70 ( 10% of the total Mn by com ...
CHAPTER 8 Structures and nomenclature of organic
... secondary, tertiary), carboxylic acids and non-branched ...
... secondary, tertiary), carboxylic acids and non-branched ...
sec chemistry may 2011 marking scheme
... A compound that contains only carbon and hydrogen atoms. • Carbon can catenate / form chains of C atoms • An atom of carbon can form stable (or strong) covalent bonds with other carbon atoms. Gases (or fuel gas) (Do not accept LPG) Petrol (or gasoline) / naphtha Any two from: • different sized molec ...
... A compound that contains only carbon and hydrogen atoms. • Carbon can catenate / form chains of C atoms • An atom of carbon can form stable (or strong) covalent bonds with other carbon atoms. Gases (or fuel gas) (Do not accept LPG) Petrol (or gasoline) / naphtha Any two from: • different sized molec ...
Identification of Aspartic and Isoaspartic Acid Residues in Amyloid β
... Asp isomerization;32,33 however, this can only be applied to detection of modification sites in the protein, but not to identify modifications already existing in biological samples prior the analysis. In addition, Alfaro et al. recently introduced a new method for the affinity enrichment of isoaspa ...
... Asp isomerization;32,33 however, this can only be applied to detection of modification sites in the protein, but not to identify modifications already existing in biological samples prior the analysis. In addition, Alfaro et al. recently introduced a new method for the affinity enrichment of isoaspa ...
Supporting Information - Royal Society of Chemistry
... absorbance followed at 450 nm). The kinetic data were analyzed via the double reciprocal plots of the initial rates of the enzyme catalyses and the substrate concentrations in the presence of different concentrations of inhibitors. For clarity, such plots are shown only for selected concentrations o ...
... absorbance followed at 450 nm). The kinetic data were analyzed via the double reciprocal plots of the initial rates of the enzyme catalyses and the substrate concentrations in the presence of different concentrations of inhibitors. For clarity, such plots are shown only for selected concentrations o ...
Ch#1 Introduction - Seattle Central College
... a clear distinction between science and philosophy. ...
... a clear distinction between science and philosophy. ...
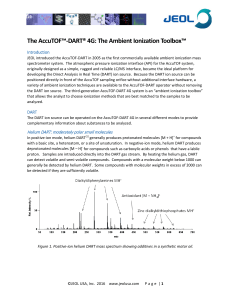
The Ambient Ionization Toolbox
... positioned directly in front of the AccuTOF sampling orifice without additional interface hardware, a variety of ambient ionization techniques are available to the AccuTOF-DART operator without removing the DART ion source. The third-generation AccuTOF-DART 4G system is an “ambient ionization toolbo ...
... positioned directly in front of the AccuTOF sampling orifice without additional interface hardware, a variety of ambient ionization techniques are available to the AccuTOF-DART operator without removing the DART ion source. The third-generation AccuTOF-DART 4G system is an “ambient ionization toolbo ...
ExamView Pro - Chapter 03.bnk
... 3) The contents of test tube #1 can be broken down into subunits that are all exactly identical to each other. 4) The macromolecule in test tube #2 is found to have a globular shape. What are the identities of the macromolecules present in the four test tubes? Write your answer in the ...
... 3) The contents of test tube #1 can be broken down into subunits that are all exactly identical to each other. 4) The macromolecule in test tube #2 is found to have a globular shape. What are the identities of the macromolecules present in the four test tubes? Write your answer in the ...
No Slide Title
... 2 atoms Mg + 1 molecule O2 makes 2 formula units MgO 2 moles Mg + 1 mole O2 makes 2 moles MgO 48.6 grams Mg + 32.0 grams O2 makes 80.6 g MgO ...
... 2 atoms Mg + 1 molecule O2 makes 2 formula units MgO 2 moles Mg + 1 mole O2 makes 2 moles MgO 48.6 grams Mg + 32.0 grams O2 makes 80.6 g MgO ...
Carbonyl Compounds notes
... it can be used to make pharmaceuticals and cosmetics. So the alkaline hydrolysis of fats and oils produces soap, which usually has the formula C17H35COO-Na+. Glycerol is also formed as a useful by-product. Soap molecules have a polar end, which is the carboxylate end, which attracts water and other ...
... it can be used to make pharmaceuticals and cosmetics. So the alkaline hydrolysis of fats and oils produces soap, which usually has the formula C17H35COO-Na+. Glycerol is also formed as a useful by-product. Soap molecules have a polar end, which is the carboxylate end, which attracts water and other ...
1990-Spring-Exam-2-student
... a) Write the structural formula(s) of the organic product(s) expected; if anything can be said about relative amounts or products, assuming more than one organic product is formed, so indicate. ...
... a) Write the structural formula(s) of the organic product(s) expected; if anything can be said about relative amounts or products, assuming more than one organic product is formed, so indicate. ...
Net ionic equation
... somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a partial negative charge on the oxygen atom (-) and partial positive charges on the hydrogen atoms (+), where “” indicates a small positive ...
... somewhat surprising that ionic compounds will dissolve in water. The reason some ionic compounds will dissolve in water is because the water molecules have a partial negative charge on the oxygen atom (-) and partial positive charges on the hydrogen atoms (+), where “” indicates a small positive ...
Answer Key, Problem Set 6 – complete, with explanations
... ions, I have shown the ions as “touching” here—you could have shown them with a bit of space in between them as well, as long as the amount of space in between was roughly “equal” for all adjacent ions). To further ...
... ions, I have shown the ions as “touching” here—you could have shown them with a bit of space in between them as well, as long as the amount of space in between was roughly “equal” for all adjacent ions). To further ...