
Chemistry - Swami Ramanand Teerth Marathwada University
... a) Introduction: Definition of qualitative analysis, macro, micro and semimicro qualitative analysis, radicals, acidic and basic radicals. b) Role of sodium carbonate extract in qualitative analysis. Interfering radicals. Removal of interfering radicals such as oxalate, c) borate, fluoride and phosp ...
... a) Introduction: Definition of qualitative analysis, macro, micro and semimicro qualitative analysis, radicals, acidic and basic radicals. b) Role of sodium carbonate extract in qualitative analysis. Interfering radicals. Removal of interfering radicals such as oxalate, c) borate, fluoride and phosp ...
Chem. 1A Week 11 Discussion Notes Dr. Mack/S12 Page 1 of 5 B
... None of the atoms in structures 5 or 6 has a formal charge, so these two structures are predicted to be stable. In this case, structures 5 and 6 are said to be “isomers,” both with the molecular formula C2H6O. (Structure 5 is ethyl alcohol and structure 6 is dimethyl ether.) None of the atoms in ...
... None of the atoms in structures 5 or 6 has a formal charge, so these two structures are predicted to be stable. In this case, structures 5 and 6 are said to be “isomers,” both with the molecular formula C2H6O. (Structure 5 is ethyl alcohol and structure 6 is dimethyl ether.) None of the atoms in ...
Organic-IB-Short-Exam Questions-Answers
... Ethene, propene and but-2-ene are members of the alkene homologous series. (a) ...
... Ethene, propene and but-2-ene are members of the alkene homologous series. (a) ...
Course Home - Haldia Institute of Technology
... implications, and become familiar with their use and applications. FT301.3 Ability to predict intermolecular potential and excess property behavior of multi component systems and ability to determine rate constant of different reactions. FT301.4 Ability to understand physical transformations in pure ...
... implications, and become familiar with their use and applications. FT301.3 Ability to predict intermolecular potential and excess property behavior of multi component systems and ability to determine rate constant of different reactions. FT301.4 Ability to understand physical transformations in pure ...
Structural Organic Chemistry The Shapes of Molecules Functional
... two bonded atoms, regardless of where the bonding electrons happen to be. The customary unit of length is the angstrom1 (A = 10-lo m), and measurements often can be made with an accuracy of 0.001 A by using the techniques of molecular spectroscopy, x-ray diffraction (for crystalline solids), and ele ...
... two bonded atoms, regardless of where the bonding electrons happen to be. The customary unit of length is the angstrom1 (A = 10-lo m), and measurements often can be made with an accuracy of 0.001 A by using the techniques of molecular spectroscopy, x-ray diffraction (for crystalline solids), and ele ...
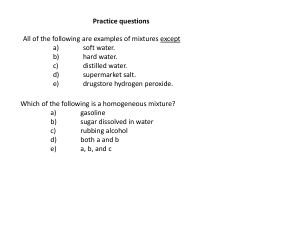
Practice questions
... There are three isotopes of carbon differing with respect to a) neutrons. b) atomic number. c) nuclear charge. d) electron configuration. e) number of protons. ...
... There are three isotopes of carbon differing with respect to a) neutrons. b) atomic number. c) nuclear charge. d) electron configuration. e) number of protons. ...
Reactions (The Basics)
... Four abbreviations are used to indicate physical states of chemicals: shown as subscripts in the chemical equation ...
... Four abbreviations are used to indicate physical states of chemicals: shown as subscripts in the chemical equation ...
Organic Chemistry - Madison Public Schools
... • Unlike alkanes, alkenes cannot rotate freely about the double bond. Side-to-side overlap makes this impossible without breaking -bond. ...
... • Unlike alkanes, alkenes cannot rotate freely about the double bond. Side-to-side overlap makes this impossible without breaking -bond. ...
Chapter 2: You must understand chemistry to understand life (and to
... A. recall that electrons in the outermost shell of an atom (valence electrons) determine the chemical behavior of the atom, i.e. what type and how many chemical bonds it can readily form B. most atoms in biological systems seek to have 8 electrons in their outermost shell (hydrogen seeks to have 0 o ...
... A. recall that electrons in the outermost shell of an atom (valence electrons) determine the chemical behavior of the atom, i.e. what type and how many chemical bonds it can readily form B. most atoms in biological systems seek to have 8 electrons in their outermost shell (hydrogen seeks to have 0 o ...
High School Curriculum Standards: Chemistry
... Chemistry is the study of matter—its properties and its changes. The idea that matter is made up of particles is over 2000 years old, but the idea of using properties of these particles to explain observable characteristics of matter has more recent origins. In ancient Greece, it was proposed that m ...
... Chemistry is the study of matter—its properties and its changes. The idea that matter is made up of particles is over 2000 years old, but the idea of using properties of these particles to explain observable characteristics of matter has more recent origins. In ancient Greece, it was proposed that m ...
化学中常见英语单词或词组的英文翻译
... A substance formed by the chemical combination of two or more ele ments. Concentration The relative abundance of a solute in a solution. Congeners Elements with similar properties, found in one column of the periodic table. Conjugate An acid and base that are related by removing or adding a single h ...
... A substance formed by the chemical combination of two or more ele ments. Concentration The relative abundance of a solute in a solution. Congeners Elements with similar properties, found in one column of the periodic table. Conjugate An acid and base that are related by removing or adding a single h ...
Working with solutions
... solute leave each other and become surrounded by particles of the solvent. O Ionic solids in water- positive and negative ions are attracted to polar water molecules. O Molecular solids in water- break up into individual neutral molecules and are surrounded by water. O Ionic compounds conducted elec ...
... solute leave each other and become surrounded by particles of the solvent. O Ionic solids in water- positive and negative ions are attracted to polar water molecules. O Molecular solids in water- break up into individual neutral molecules and are surrounded by water. O Ionic compounds conducted elec ...
Organic Reactions
... • Reactions occur between electrophiles and nucleophiles. • Just about any part of a molecule can be either electrophilic or nucleophilic depending one what you are comparing it to ...
... • Reactions occur between electrophiles and nucleophiles. • Just about any part of a molecule can be either electrophilic or nucleophilic depending one what you are comparing it to ...
Unit Description - Honors Chemistry
... Chapters 1 and 3 – Scientific Method and Matter Distinguish among hypothesis, theory and scientific law using examples. Identify the common steps of scientific methods. Distinguish between qualitative and quantitative data. Distinguish between independent and dependent variables, controls an ...
... Chapters 1 and 3 – Scientific Method and Matter Distinguish among hypothesis, theory and scientific law using examples. Identify the common steps of scientific methods. Distinguish between qualitative and quantitative data. Distinguish between independent and dependent variables, controls an ...
Esters - Phillips Scientific Methods
... FYI- Back to Biology: The below molecule is produced in the first step of Glycolysis in Cellular Respiration (oxidation of glucose), as ATP ADP to start the process, and the phosphate bonds ...
... FYI- Back to Biology: The below molecule is produced in the first step of Glycolysis in Cellular Respiration (oxidation of glucose), as ATP ADP to start the process, and the phosphate bonds ...
Ch 4 Carbon Notes
... Concept 4.2: Carbon atoms can form diverse molecules by bonding to four other atoms • Electron configuration is the key to an atom’s characteristics • Electron configuration determines the kinds and number of bonds an atom will form with other atoms ...
... Concept 4.2: Carbon atoms can form diverse molecules by bonding to four other atoms • Electron configuration is the key to an atom’s characteristics • Electron configuration determines the kinds and number of bonds an atom will form with other atoms ...
Name: Date: Block:______ GRADE 8 SCIENCE SOL QUESTIONS
... a. base reaches absolute zero b. acid evaporates c. base chemically reacts with the acid d. mass of the solution increases 3. Because zinc can combine with other substances but cannot be changed into a simpler substance by an ordinary chemical process, zinc is classified as — a. a compound b. a mixt ...
... a. base reaches absolute zero b. acid evaporates c. base chemically reacts with the acid d. mass of the solution increases 3. Because zinc can combine with other substances but cannot be changed into a simpler substance by an ordinary chemical process, zinc is classified as — a. a compound b. a mixt ...
chemistry a-level - St Thomas More High School
... Amines, Polymers, Amino Acids, Proteins and DNA, Organic Synthesis, NMR spectroscopy, Chromatography ...
... Amines, Polymers, Amino Acids, Proteins and DNA, Organic Synthesis, NMR spectroscopy, Chromatography ...
Document
... From these seven base units, several other units are derived, which means the derived units can be defined in terms of the base units, for example, liter is derived from m3. SI prefix can be attached to the names of the base units to express multiples or submultiples of these units. Using prefixes w ...
... From these seven base units, several other units are derived, which means the derived units can be defined in terms of the base units, for example, liter is derived from m3. SI prefix can be attached to the names of the base units to express multiples or submultiples of these units. Using prefixes w ...



















![Newtechniques[1]](http://s1.studyres.com/store/data/008290568_1-ce9574822ee68efb0424b4d58f36c344-300x300.png)


