Supporting Text S1.
... positive. Therefore it is possible that (GSh GTu ) changes sign on increasing temperature, as shown schematically in Fig. S3a. The sign change of (GSh GTu ) upon increasing temperature implies the entropy change (SSh STu 0) . Another possible source of entropy change is through liberat ...
... positive. Therefore it is possible that (GSh GTu ) changes sign on increasing temperature, as shown schematically in Fig. S3a. The sign change of (GSh GTu ) upon increasing temperature implies the entropy change (SSh STu 0) . Another possible source of entropy change is through liberat ...
Document
... • There will be two possible Wittig routes to an alkene. • Analyze the structure retrosynthetically, i.e., work the synthesis out backworks • Disconnect (break the bond of the target that can be formed by a known reaction) the doubly bonded carbons. One becomes the aldehyde or ketone, the other the ...
... • There will be two possible Wittig routes to an alkene. • Analyze the structure retrosynthetically, i.e., work the synthesis out backworks • Disconnect (break the bond of the target that can be formed by a known reaction) the doubly bonded carbons. One becomes the aldehyde or ketone, the other the ...
Developing Lewis structures for organic molecules 1) Draw the full
... that lack a full octet (usually heteroatoms in organic molecules) as lone pairs. 4) Atoms* lacking octets are completed by using lone pairs from adjacent atoms to form multiple bonds. Where more than one such pair is available, then all possibilities must be drawn and evaluated: these are resonance ...
... that lack a full octet (usually heteroatoms in organic molecules) as lone pairs. 4) Atoms* lacking octets are completed by using lone pairs from adjacent atoms to form multiple bonds. Where more than one such pair is available, then all possibilities must be drawn and evaluated: these are resonance ...
Chapter 1 Matter and Energy Classifying Matter – An Exercise
... Practice Solutions Representations of Matter Metals can be distinguished from nonmetals by the luster and ability to conduct electricity. Since we do not know how each of elements in Figure 1.4 conduct electricity, we need to use luster as our measure. Nonmetals are usually dull, with the exception ...
... Practice Solutions Representations of Matter Metals can be distinguished from nonmetals by the luster and ability to conduct electricity. Since we do not know how each of elements in Figure 1.4 conduct electricity, we need to use luster as our measure. Nonmetals are usually dull, with the exception ...
Stoichiometry/Mass/Mole Relationships
... 10. ___ C6H12 + ___ O2 → ___ CO2 + ___ H2O 42 grams of cyclohexane burns in excess air to from carbon dioxide and water. How many grams of carbon dioxide and of water vapor are produced? ...
... 10. ___ C6H12 + ___ O2 → ___ CO2 + ___ H2O 42 grams of cyclohexane burns in excess air to from carbon dioxide and water. How many grams of carbon dioxide and of water vapor are produced? ...
Chapter 5 Alt Notes 0910
... Thermodynamics is the study of the changes in energy and transfers of energy that accompany chemical and physical processes. In this chapter we will address 3 fundamental questions. Will two (or more) substances react when they are mixed under specified conditions? If they do react, what energy chan ...
... Thermodynamics is the study of the changes in energy and transfers of energy that accompany chemical and physical processes. In this chapter we will address 3 fundamental questions. Will two (or more) substances react when they are mixed under specified conditions? If they do react, what energy chan ...
Topic 13 – Biomolecules
... Globular these tend to be the working proteins and include enzymes and antibodies Conjugated these proteins are associated with a non protein component for example haemoglobin which is associated with iron. ...
... Globular these tend to be the working proteins and include enzymes and antibodies Conjugated these proteins are associated with a non protein component for example haemoglobin which is associated with iron. ...
Chapter 9: Molecular Geometry and Hybridization of Atomic Orbitals
... Now, there are two orbitals in the Be-atom available for bonding. One Cl-atom would share the 2s orbital, and the other Cl-atom would share the 2p orbital. This will result in two non-equivalent Be-Cl bonds. However, experiments suggest that the two Be-Cl bonds are equivalent in every respect. ...
... Now, there are two orbitals in the Be-atom available for bonding. One Cl-atom would share the 2s orbital, and the other Cl-atom would share the 2p orbital. This will result in two non-equivalent Be-Cl bonds. However, experiments suggest that the two Be-Cl bonds are equivalent in every respect. ...
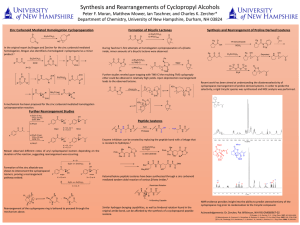
Here is the Original File - University of New Hampshire
... [1] Brogan , J. B.; Zercher, C. K. J. Org. Chem. 1997, 62, 6444-6446. [2] Mastrolorenzo, A.; Ruscouni, S.; Scozzafava, A.; Barbaro, G.; Supran, C.T. Curr. Med. Chem. 2007, I14I, 2734-2748 [3] Lin, W.; Therberge, C.R.; Henderson, T. J.; Zercher C. K.; Jasinski, J.; Butcher, R. J. J. Org. Chem. 2009, ...
... [1] Brogan , J. B.; Zercher, C. K. J. Org. Chem. 1997, 62, 6444-6446. [2] Mastrolorenzo, A.; Ruscouni, S.; Scozzafava, A.; Barbaro, G.; Supran, C.T. Curr. Med. Chem. 2007, I14I, 2734-2748 [3] Lin, W.; Therberge, C.R.; Henderson, T. J.; Zercher C. K.; Jasinski, J.; Butcher, R. J. J. Org. Chem. 2009, ...
Answers
... 6. Draw the acetal products that form when ethanol is condensed with each of these aldehydes/ketones and catalytic acid. ...
... 6. Draw the acetal products that form when ethanol is condensed with each of these aldehydes/ketones and catalytic acid. ...
CHM 110 - Equation Interpretation (r14)
... Thus, for every 0.345 moles of iron, you can produce 0.173 moles of Fe2O3. You've just learned the heart of stoichiometry. By putting this together with your knowledge of the way to relate mass and moles (via the formula weight), you can solve more complex stoichiometry problems. Summary In this not ...
... Thus, for every 0.345 moles of iron, you can produce 0.173 moles of Fe2O3. You've just learned the heart of stoichiometry. By putting this together with your knowledge of the way to relate mass and moles (via the formula weight), you can solve more complex stoichiometry problems. Summary In this not ...
Chemistry Final Exam Review
... • basic characteristics and names of the major groups • metals, nonmetals, metalloids – “staircase” • ionization energy, electronegativity, atomic radius, trends shown in these properties on the periodic table Problems: 1. Give the number of valence electrons, physical state (metal, nonmetal, or met ...
... • basic characteristics and names of the major groups • metals, nonmetals, metalloids – “staircase” • ionization energy, electronegativity, atomic radius, trends shown in these properties on the periodic table Problems: 1. Give the number of valence electrons, physical state (metal, nonmetal, or met ...
AP Chemistry Summer Assignment
... flashcards for the polyatomic ions that you must learn. I strongly suggest that you cut them out and begin memorizing them immediately. Use the hints on the common ions sheet to help you reduce the amount of memorizing that you must do. The 7 diatomic molecules (HOFBrINCl) **There will be a test on ...
... flashcards for the polyatomic ions that you must learn. I strongly suggest that you cut them out and begin memorizing them immediately. Use the hints on the common ions sheet to help you reduce the amount of memorizing that you must do. The 7 diatomic molecules (HOFBrINCl) **There will be a test on ...
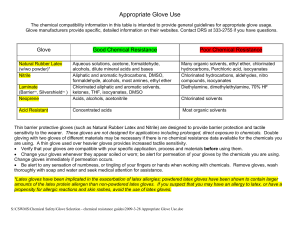
Appropriate Glove Use
... alcohols, dilute mineral acids and bases Aliphatic and aromatic hydrocarbons, DMSO, formaldehyde, alcohols, most amines, ethyl ether Chlorinated aliphatic and aromatic solvents, ketones, THF, isocyanates, DMSO Acids, alcohols, acetonitrile ...
... alcohols, dilute mineral acids and bases Aliphatic and aromatic hydrocarbons, DMSO, formaldehyde, alcohols, most amines, ethyl ether Chlorinated aliphatic and aromatic solvents, ketones, THF, isocyanates, DMSO Acids, alcohols, acetonitrile ...
CHEMISTRY 132
... All notes, books, etc., must be placed out of sight. Please read each of the problems carefully. If something is not clear, please ask. The exam is worth a total of 108 points. Make sure your exam is complete. A periodic table is attached as the last sheet on the exam. Answers should be placed in th ...
... All notes, books, etc., must be placed out of sight. Please read each of the problems carefully. If something is not clear, please ask. The exam is worth a total of 108 points. Make sure your exam is complete. A periodic table is attached as the last sheet on the exam. Answers should be placed in th ...
unit c3 – chemistry in action checklist
... Define homologous series as a series of compounds which: a have the same general formula b show a gradual variation in physical properties as exemplified by their boiling points c have similar chemical properties Recall the names, formulae and structures of members of the following homologous series ...
... Define homologous series as a series of compounds which: a have the same general formula b show a gradual variation in physical properties as exemplified by their boiling points c have similar chemical properties Recall the names, formulae and structures of members of the following homologous series ...
Topic 1 Assignment File
... bromine. The following equation is an example of this reaction. KBr + Cl2 -‐-‐-‐-‐ KCl + Br2 When 0.855 mole of Cl2 and 3.305 g of KBr are mixed in ...
... bromine. The following equation is an example of this reaction. KBr + Cl2 -‐-‐-‐-‐ KCl + Br2 When 0.855 mole of Cl2 and 3.305 g of KBr are mixed in ...