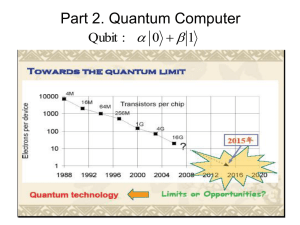
computational chemistry
... available and when to use them. Prioritizing which techniques work better or worse for various types of problems is a double-edged sword. This is certainly the type of information that is of use in solving practical problems, but there is no rigorous mathematical way to prove which techniques work b ...
... available and when to use them. Prioritizing which techniques work better or worse for various types of problems is a double-edged sword. This is certainly the type of information that is of use in solving practical problems, but there is no rigorous mathematical way to prove which techniques work b ...
Fall - Physical Chemistry Division
... dimensionality starting from 0D fullerenes, to 1D carbon nanotubes (CNTs) and graphene nanoribbons (GNRs), 2D single-layered (or few layered) graphene, up to 3D graphite, their derivatives, and intercalated compounds. Graphitic materials are interesting both from a basic research viewpoint and for i ...
... dimensionality starting from 0D fullerenes, to 1D carbon nanotubes (CNTs) and graphene nanoribbons (GNRs), 2D single-layered (or few layered) graphene, up to 3D graphite, their derivatives, and intercalated compounds. Graphitic materials are interesting both from a basic research viewpoint and for i ...
Derivation and Experimental Proof of the Universal Force
... current pillars of modern physics, i.e. electrodynamics, quantum mechanics, and relativity theory are incomplete and incompatible with one another. These theories were developed under the philosophy of Existentialism which is based upon idealizations instead of reality. This work shows how to perfec ...
... current pillars of modern physics, i.e. electrodynamics, quantum mechanics, and relativity theory are incomplete and incompatible with one another. These theories were developed under the philosophy of Existentialism which is based upon idealizations instead of reality. This work shows how to perfec ...
Chapter 7. Statistical Mechanics page 491
... 1. web links to home pages of a multitude of practicing theoretical chemists who specialize in many of the topics discussed in this text; 2. numerous education-site web links that allow students ranging from fresh-persons to advanced graduate students to seek out a variety of information; 3. textual ...
... 1. web links to home pages of a multitude of practicing theoretical chemists who specialize in many of the topics discussed in this text; 2. numerous education-site web links that allow students ranging from fresh-persons to advanced graduate students to seek out a variety of information; 3. textual ...
Study Guide for Ch. 1
... Identify the benefits of the metric system versus classical measurement. Temperature scales and their details. Differentiate between solutions, colloids, and suspensions. Understand the physical properties involved in determining solids, liquids, & gases. Use significant figures in calculations and ...
... Identify the benefits of the metric system versus classical measurement. Temperature scales and their details. Differentiate between solutions, colloids, and suspensions. Understand the physical properties involved in determining solids, liquids, & gases. Use significant figures in calculations and ...
Importance of Molecular Simulation for Studying Structural Properties
... computational techniques used to model or mimic the behavior of molecules. The techniques are used in the fields of computational chemistry, drug design, computational biology and materials science for studying molecular systems ranging from small chemical systems to large biological molecules and m ...
... computational techniques used to model or mimic the behavior of molecules. The techniques are used in the fields of computational chemistry, drug design, computational biology and materials science for studying molecular systems ranging from small chemical systems to large biological molecules and m ...
Subject Group of Applied Physics
... in quantum theory in order to explain various interesting phenomena reflecting the nature of the electron as a wave, or not as a particle. Only a few assumptions on each problem setup lead to a variety of quantum interference physics, for example, electronic states confined in low-dimensional system ...
... in quantum theory in order to explain various interesting phenomena reflecting the nature of the electron as a wave, or not as a particle. Only a few assumptions on each problem setup lead to a variety of quantum interference physics, for example, electronic states confined in low-dimensional system ...
BIG IDEAS - BC Curriculum - Province of British Columbia
... • Demonstrate an awareness of assumptions, question information given, and identify bias in their own work and in primary and secondary sources • Consider the changes in knowledge over time as tools and technologies have developed • Connect scientific explorations to careers in science • Exercise a ...
... • Demonstrate an awareness of assumptions, question information given, and identify bias in their own work and in primary and secondary sources • Consider the changes in knowledge over time as tools and technologies have developed • Connect scientific explorations to careers in science • Exercise a ...
741-Dr. Janadeh
... The radar and radio waves are used to follow flip motion of spin of electrons and protons, respectively. These lead to electron spin resonance (ESR) and nuclear magnetic resonance (NMR) spectroscopy, respectively. The field of science that deals with wave motion of systems like electrons, atoms, mol ...
... The radar and radio waves are used to follow flip motion of spin of electrons and protons, respectively. These lead to electron spin resonance (ESR) and nuclear magnetic resonance (NMR) spectroscopy, respectively. The field of science that deals with wave motion of systems like electrons, atoms, mol ...
2 pt 3 pt 4 pt 5pt 1 pt 2 pt 3 pt 4 pt 5 pt 1 pt 2pt 3 pt 4pt 5
... Bohr proposed that an electron is found only specific circular paths or orbits around the nucleus. ...
... Bohr proposed that an electron is found only specific circular paths or orbits around the nucleus. ...
TDDFT as a tool in chemistry and biochemistry
... B! In a truncated CI expansion (see figure) when the two systems A and B are treated as independent noninteracting moieties, they correspond to double-excited configurations.! Instead, as a joined system, (A+B) is represented as a quadruple-excitation.! The two calculations are not performed at the ...
... B! In a truncated CI expansion (see figure) when the two systems A and B are treated as independent noninteracting moieties, they correspond to double-excited configurations.! Instead, as a joined system, (A+B) is represented as a quadruple-excitation.! The two calculations are not performed at the ...
TDDFT as a tool in chemistry
... Variational (give an upper bound to the exact energy). Size consistent (especially important in chemical reactions). Correct ordering of the excited states energies. Energies and wavefunction (density) should possibly be analytically differentiable with respect to external parameters (for instance n ...
... Variational (give an upper bound to the exact energy). Size consistent (especially important in chemical reactions). Correct ordering of the excited states energies. Energies and wavefunction (density) should possibly be analytically differentiable with respect to external parameters (for instance n ...
Introduction to Computational Chemistry
... which a restricted part of reality can be understood and discussed on the basis of a minimum number of preferably simple rules. Typical examples for models that have deeply invaded chemical thinking are ...
... which a restricted part of reality can be understood and discussed on the basis of a minimum number of preferably simple rules. Typical examples for models that have deeply invaded chemical thinking are ...
Introduction to Computational Chemistry
... Computational chemists are hired by academic institutions, government agencies, and all types of industries. The pharmaceutical industry in particular has embraced computational chemistry as an effective tool in the design of new drugs. Computational chemists are employed to study all types of chemi ...
... Computational chemists are hired by academic institutions, government agencies, and all types of industries. The pharmaceutical industry in particular has embraced computational chemistry as an effective tool in the design of new drugs. Computational chemists are employed to study all types of chemi ...