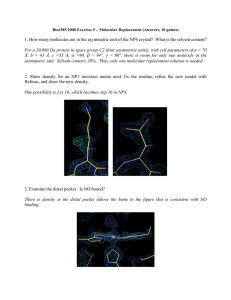
Review Packet
... a. can be broken down into simpler substances b. are used to make other elements c. are used to make compounds d. are never found in the periodic table of elements 24. Chemical reactions can be used to separate a. elements b. pure substances b. mixtures d. compounds 25. A change in the force of Eart ...
... a. can be broken down into simpler substances b. are used to make other elements c. are used to make compounds d. are never found in the periodic table of elements 24. Chemical reactions can be used to separate a. elements b. pure substances b. mixtures d. compounds 25. A change in the force of Eart ...
On the Chemistry of the Resveratrol Diastereomers
... field from ¸(bio)catalyst discovery to (bio)catalytic microprocess design. This advance will require not only a new level of understanding of reaction mechanisms and transport phenomena at the micro scale, but also the development of computational tools [1]. In this work the theoretical description ...
... field from ¸(bio)catalyst discovery to (bio)catalytic microprocess design. This advance will require not only a new level of understanding of reaction mechanisms and transport phenomena at the micro scale, but also the development of computational tools [1]. In this work the theoretical description ...
Section 2-4 “Chemical Reactions and Enzymes”
... Products – Elements or compounds produced by a chemical reaction ...
... Products – Elements or compounds produced by a chemical reaction ...
Unit 4 Evolution
... Science Starter: Copy and answer. Can a phase change produce new compounds? No, because chemical changes occur. ...
... Science Starter: Copy and answer. Can a phase change produce new compounds? No, because chemical changes occur. ...
Theoretical Calculation of Enthalpy of reactions involved in PZ
... Combining the well-known Gibbs Helmholtz equation [3] with equations 8 and 9, the enthalpy of the overall reaction can be expressed as ...
... Combining the well-known Gibbs Helmholtz equation [3] with equations 8 and 9, the enthalpy of the overall reaction can be expressed as ...
Chem 1151
... (5) Titration with solutions of KBrO3 can be used to determine the concentration of As(III) in an unknown sample. What is the molarity of As(III) if 33.45 mL of 0.125M KBrO3 is needed to titrate 50.0 mL of the As(III) solution? The balanced chemical equation is H3AsO3(aq) + BrO3-(aq) Br -(aq) + 3H ...
... (5) Titration with solutions of KBrO3 can be used to determine the concentration of As(III) in an unknown sample. What is the molarity of As(III) if 33.45 mL of 0.125M KBrO3 is needed to titrate 50.0 mL of the As(III) solution? The balanced chemical equation is H3AsO3(aq) + BrO3-(aq) Br -(aq) + 3H ...
AP Chemistry Syllabus - Tuloso
... Knowledge of specific facts of chemistry is essential for an understanding of principles and concepts. These descriptive facts, including the chemistry involved in environmental and societal issues, should not be isolated from the principles being studied but should be taught throughout the course t ...
... Knowledge of specific facts of chemistry is essential for an understanding of principles and concepts. These descriptive facts, including the chemistry involved in environmental and societal issues, should not be isolated from the principles being studied but should be taught throughout the course t ...
2A Final Exam Review Worksheet
... o Temperature is proportional to kinetic energy. Two molecules at the same temperature will have the same average kinetic energy. o If two molecules are under the same conditions, the heavier molecule will travel slower ...
... o Temperature is proportional to kinetic energy. Two molecules at the same temperature will have the same average kinetic energy. o If two molecules are under the same conditions, the heavier molecule will travel slower ...
Solutions to Nonlinear Equations
... Where F is a polynomial or a transcendental function, given explicitly. – Exact solutions are not possible for most equations. – A number x ± e, ( e > 0 ) is an approximate solution of the equation if there is a solution in the interval [x-e,x+e]. e is the maximum possible error in the approximate s ...
... Where F is a polynomial or a transcendental function, given explicitly. – Exact solutions are not possible for most equations. – A number x ± e, ( e > 0 ) is an approximate solution of the equation if there is a solution in the interval [x-e,x+e]. e is the maximum possible error in the approximate s ...
CY 101- Chemistry Atomic Structure
... •Let us test the Schrödinger equation for some simple physical systems! Some model systems at first – easy to test ! Hopefully, some quantum properties of matter will also be understood! •If we can understand these ‘simple systems’ without doing any approximation and can derive ‘exact solutions’, th ...
... •Let us test the Schrödinger equation for some simple physical systems! Some model systems at first – easy to test ! Hopefully, some quantum properties of matter will also be understood! •If we can understand these ‘simple systems’ without doing any approximation and can derive ‘exact solutions’, th ...
Chemistry Syllabus
... b. Relate the information to the concepts you are studying. Discuss the patterns of the graphs how they relate to the concept. If there is no graph, use the data to help explain the concept. Error Analysis (Sources of Error): This is the last section where you explain what errors were committed duri ...
... b. Relate the information to the concepts you are studying. Discuss the patterns of the graphs how they relate to the concept. If there is no graph, use the data to help explain the concept. Error Analysis (Sources of Error): This is the last section where you explain what errors were committed duri ...
PowerPoint Overview for Introduction
... Some of the more prominent representatives are called macronutrients, whereas those appearing only at the level of parts per million or less are referred to as micronutrients. These nutrients perform various functions, including the building of bones and cell structures, regulating the body's pH, ca ...
... Some of the more prominent representatives are called macronutrients, whereas those appearing only at the level of parts per million or less are referred to as micronutrients. These nutrients perform various functions, including the building of bones and cell structures, regulating the body's pH, ca ...
Chemistry Outcomes - hrsbstaff.ednet.ns.ca
... Distinguish between molecular and ionic compounds Use periodic table (ionic table) and polyatomic ionic table to correctly write chemical formula from a given name. Apply rules for nomenclature for ionic and molecular compounds if given chemical formula Given the name for an ionic or molecular formu ...
... Distinguish between molecular and ionic compounds Use periodic table (ionic table) and polyatomic ionic table to correctly write chemical formula from a given name. Apply rules for nomenclature for ionic and molecular compounds if given chemical formula Given the name for an ionic or molecular formu ...
... I will review some of these, including pump-probe experiments on the large molecular complexes involved in photosynthesis, and on a drug target molecule for sleeping sickness. A new approach to disentangling orientational disorder will also be demonstrated, aimed at reconstructing the image of one m ...
Z Notation
... 1. Formal methods can guarantee that software is perfect. Rather: they are very helpful at finding errors early on and can nearly eliminate some classes of error. 2. They are all about program proving. Rather: they work largely by making you think very hard about the system you propose to build. 3. ...
... 1. Formal methods can guarantee that software is perfect. Rather: they are very helpful at finding errors early on and can nearly eliminate some classes of error. 2. They are all about program proving. Rather: they work largely by making you think very hard about the system you propose to build. 3. ...
Chapter Outline Measuring Polarity Examples Permanent Dipole
... around the central C atom is tetrahedral. ...
... around the central C atom is tetrahedral. ...