atomic theory of matter
... PROPORTIONS • Some elements can form more than one compound when they react together (C & O: CO and CO2; N & O: N2O, NO, NO2, etc.). Dalton’s law predicted that the mass proportions should be proportional. Experiment confirmed this leading to this law. • Law of multiple proportions: when two element ...
... PROPORTIONS • Some elements can form more than one compound when they react together (C & O: CO and CO2; N & O: N2O, NO, NO2, etc.). Dalton’s law predicted that the mass proportions should be proportional. Experiment confirmed this leading to this law. • Law of multiple proportions: when two element ...
Developing mathematical thinking in the curriculum 2008
... • Sorting in different ways draws attention to all the things that can vary • Sorting in several ways encourages focus on properties instead of visual similarities ...
... • Sorting in different ways draws attention to all the things that can vary • Sorting in several ways encourages focus on properties instead of visual similarities ...
Chemistry EOC Review
... Chemistry EOC Review Directions: The following is an End-Of-Course Review Guide designed to assist you as prepare for your EOC. It is imperative that you complete this guide to the best of your ability. This will help you to achieve a higher average on your third quarter grade. Answer as many questi ...
... Chemistry EOC Review Directions: The following is an End-Of-Course Review Guide designed to assist you as prepare for your EOC. It is imperative that you complete this guide to the best of your ability. This will help you to achieve a higher average on your third quarter grade. Answer as many questi ...
The chemical elements are fundamental building materials of matter
... • 1.A: All matter is made of atoms. There are a limited number of types of atoms: these are the elements. • 1.B: The atoms of each element have unique structures arising from interactions between electrons and nuclei. • 1.C: Elements display periodicity in their properties when the elements are orga ...
... • 1.A: All matter is made of atoms. There are a limited number of types of atoms: these are the elements. • 1.B: The atoms of each element have unique structures arising from interactions between electrons and nuclei. • 1.C: Elements display periodicity in their properties when the elements are orga ...
Viju B - IS MU
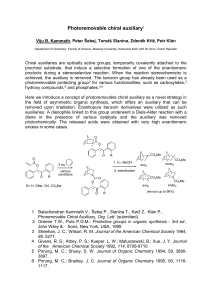
... products during a stereoselective reaction. When the reaction stereochemistry is achieved, the auxiliary is removed. The benzoin group has already been used as a photoremovable protecting group2 for various functionalities, such as carboxylates,3 hydroxy compounds,6 and phosphates.4,5 Here we introd ...
... products during a stereoselective reaction. When the reaction stereochemistry is achieved, the auxiliary is removed. The benzoin group has already been used as a photoremovable protecting group2 for various functionalities, such as carboxylates,3 hydroxy compounds,6 and phosphates.4,5 Here we introd ...
Chemistry EOC Review Spring 2013
... Unit 1 & 2 Lab Safety, Introduction to Chemistry, & Measurement (Chapters 1 & 3): 1. What is chemistry? 2. Distinguish between hypothesis, theory, and law. 3. Classify the following as having good or poor accuracy and good or poor precision: 4. A scientist experimentally determines the speed of ligh ...
... Unit 1 & 2 Lab Safety, Introduction to Chemistry, & Measurement (Chapters 1 & 3): 1. What is chemistry? 2. Distinguish between hypothesis, theory, and law. 3. Classify the following as having good or poor accuracy and good or poor precision: 4. A scientist experimentally determines the speed of ligh ...
chem eng-problems
... 2. Magnesium has three naturally occuring isotopes. 78.70% of Magnesium atoms exist as Magnesium-24 (23.9850 g/mol), 10.03% exist as Magnesium-25 (24.9858 g/mol) and 11.17% exist as Magnesium-26 (25.9826 g/mol). What is the average atomic mass of Magnesium? ...
... 2. Magnesium has three naturally occuring isotopes. 78.70% of Magnesium atoms exist as Magnesium-24 (23.9850 g/mol), 10.03% exist as Magnesium-25 (24.9858 g/mol) and 11.17% exist as Magnesium-26 (25.9826 g/mol). What is the average atomic mass of Magnesium? ...
104 Homework Packet - Rogue Community College
... According to Le Chatelier’s Principle, adding reactants (or removing products) drives the equilibrium to the __________, adding products (or removing reactants) drives the equilibrium to the __________, increasing temperature favors the ___________________ reaction, decreasing temperature favors the ...
... According to Le Chatelier’s Principle, adding reactants (or removing products) drives the equilibrium to the __________, adding products (or removing reactants) drives the equilibrium to the __________, increasing temperature favors the ___________________ reaction, decreasing temperature favors the ...
Midterm Review Date
... B) low ionization energy and high electronegativity C) high ionization energy and low electronegativity D) high ionization energy and high electronegativity 22. Which element is an alkali metal? ...
... B) low ionization energy and high electronegativity C) high ionization energy and low electronegativity D) high ionization energy and high electronegativity 22. Which element is an alkali metal? ...
PDF - World Journal of Pharmaceutical Sciences
... national and international peer-reviewed journals of high impact factor and editorial board member/advisor of 40 international peer-reviewed journals. He is the author of 160 abstracts of national/international conferences and also the author of 16 books. He has guided 42 MPharm research projects an ...
... national and international peer-reviewed journals of high impact factor and editorial board member/advisor of 40 international peer-reviewed journals. He is the author of 160 abstracts of national/international conferences and also the author of 16 books. He has guided 42 MPharm research projects an ...
Name Date: __ ______ Chemistry Semester I Final Exam Review
... 15. What is a chemical change? _______________________________________________________________ Which of the following is a chemical change: ripping a piece of paper, melting ice, burning wood? 16. Give 2 examples of physical changes: _______________________________________________________ 17. What i ...
... 15. What is a chemical change? _______________________________________________________________ Which of the following is a chemical change: ripping a piece of paper, melting ice, burning wood? 16. Give 2 examples of physical changes: _______________________________________________________ 17. What i ...
Chemistry - Delhi Public School, Faridabad
... Carbon has electronic configuration 1s 2 2s2 2p2 and therefore, should be bivalent. How will you justify its tetravalency in methane? ...
... Carbon has electronic configuration 1s 2 2s2 2p2 and therefore, should be bivalent. How will you justify its tetravalency in methane? ...
Bonding and Nomenclature
... • Same as last except… • Step #4: If there are no electrons left, move electrons from a different atom to form another bond…double • Side note: When more than one Lewis structure can be drawn, the molecule or ion is said to have resonance. ...
... • Same as last except… • Step #4: If there are no electrons left, move electrons from a different atom to form another bond…double • Side note: When more than one Lewis structure can be drawn, the molecule or ion is said to have resonance. ...
research project
... Formation and transformations of bonds, interconversion of functional groups of easily starting materials into complex building blocks is very important in Organic Chemistry. ...
... Formation and transformations of bonds, interconversion of functional groups of easily starting materials into complex building blocks is very important in Organic Chemistry. ...
Using mass to calculate molecular formula
... Empirical formula and Molecular formula Benzene consists of 7.69% H and 92.31%C. Converting this to a formula gives CH. This is the simplest integer ratio. In fact a molecule of benzene has the formula C6H6. Empirical formula CH – simplest whole number ratio. Molecular formula C6H6 – actual number o ...
... Empirical formula and Molecular formula Benzene consists of 7.69% H and 92.31%C. Converting this to a formula gives CH. This is the simplest integer ratio. In fact a molecule of benzene has the formula C6H6. Empirical formula CH – simplest whole number ratio. Molecular formula C6H6 – actual number o ...
Chemical Reactions Chemical Arithmetic
... reaction in which the oxidation numbers of elements change because of a loss or gain of electrons • Oxidation Number- A number that indicates the charge that an atom in a molecule or polyatomic ion would have if all bonds were ionic. – Fictitious- No actual charge of this magnitude actually exists w ...
... reaction in which the oxidation numbers of elements change because of a loss or gain of electrons • Oxidation Number- A number that indicates the charge that an atom in a molecule or polyatomic ion would have if all bonds were ionic. – Fictitious- No actual charge of this magnitude actually exists w ...
Molecular Geometry Why?
... A lone pair of electrons and a bonded pair of electrons will (push away from/move toward) each other. ...
... A lone pair of electrons and a bonded pair of electrons will (push away from/move toward) each other. ...
Chapter 10. Chemical Bonding II. Molecular Geometry and
... 5. Maximum of 2 electrons per MO (with opposite spins) 6. Follow Hund's rule - electrons do not pair until all MO's of the same energy are half filled 7. The number of electrons in MOs equals sum of all of the electrons in the atoms B. MO's for 2nd Row Diatomic Molecules (e.g., N2, O2, F2, etc.) MO ...
... 5. Maximum of 2 electrons per MO (with opposite spins) 6. Follow Hund's rule - electrons do not pair until all MO's of the same energy are half filled 7. The number of electrons in MOs equals sum of all of the electrons in the atoms B. MO's for 2nd Row Diatomic Molecules (e.g., N2, O2, F2, etc.) MO ...