Chemistry 111 Study Sheet - Answers
... Melting point and freezing point are the same temperature. What is vapor pressure? The pressure of vapor in equilibrium with its liquid state. The pressure of a vapor evaporating from the liquid state. What is boiling? The especially rapid evaporation that occurs when the vapor pressure of a liquid ...
... Melting point and freezing point are the same temperature. What is vapor pressure? The pressure of vapor in equilibrium with its liquid state. The pressure of a vapor evaporating from the liquid state. What is boiling? The especially rapid evaporation that occurs when the vapor pressure of a liquid ...
CHEM 2: Exam 3
... PV=nRT, where R = 0.08206 L·atm/mol·K = 62.37 L·torr/mol·K = 8.314 L·kPa/mol·K Temperature Conversion: Kelvin = oC + 273.15 Pressure Conversions: 1 atm = 14.7 psi = 760 mmHg = 101.325 kPa = 760 torr At STP: Pressure = 1 atm, Temperature = 273 K & 1 mole of gas = 22.4 L ...
... PV=nRT, where R = 0.08206 L·atm/mol·K = 62.37 L·torr/mol·K = 8.314 L·kPa/mol·K Temperature Conversion: Kelvin = oC + 273.15 Pressure Conversions: 1 atm = 14.7 psi = 760 mmHg = 101.325 kPa = 760 torr At STP: Pressure = 1 atm, Temperature = 273 K & 1 mole of gas = 22.4 L ...
Paper
... concentration of 9.8*1014 molecules/cm3. The discrepancy can be attributed to the sealing process of the cells extracting excess water from the cell walls. The low concentration profile is shown in Figure 4. The measured concentration was 6.5*1014 molecules/cm3 with a theoretical concentration of 2. ...
... concentration of 9.8*1014 molecules/cm3. The discrepancy can be attributed to the sealing process of the cells extracting excess water from the cell walls. The low concentration profile is shown in Figure 4. The measured concentration was 6.5*1014 molecules/cm3 with a theoretical concentration of 2. ...
Unit 9: Atomic Structure, Periodicity and Chemical Bonding
... (a) potassium’s valence electron is 4s while lithium’s is 2s . potassium’s electron is shielded by more electrons than lithium and is therefore more easily removed at a lower energy. (b) The addition of electrons to a neutral atom produces an anion that is significantly larger than its parent atom. ...
... (a) potassium’s valence electron is 4s while lithium’s is 2s . potassium’s electron is shielded by more electrons than lithium and is therefore more easily removed at a lower energy. (b) The addition of electrons to a neutral atom produces an anion that is significantly larger than its parent atom. ...
Chemistry EOC Review
... 34. How are frequency and wavelength related? 35. Calculate the wavelength of a yellow light by a sodium lamp if the frequency of the radiation is 3.34 x 10 14 Hz. ...
... 34. How are frequency and wavelength related? 35. Calculate the wavelength of a yellow light by a sodium lamp if the frequency of the radiation is 3.34 x 10 14 Hz. ...
Chemistry EOC Review Spring 2013
... 12. Calculate the density of a 5.0 g object that has a volume of 2.0 cm3. 13. Convert the following temperatures: a. 34C to K b. 50 K to C ...
... 12. Calculate the density of a 5.0 g object that has a volume of 2.0 cm3. 13. Convert the following temperatures: a. 34C to K b. 50 K to C ...
Electricity & Optics Physics 24100 Lecture 14 – Chapter 27 sec. 1-2
... Pick a coordinate system Label source and field points Pick variables to express their components Express 'ℓ, 7 and 7 using these variables Evaluate 'ℓ × 7 Write out the integral for each component Evaluate the integrals one way or another. ...
... Pick a coordinate system Label source and field points Pick variables to express their components Express 'ℓ, 7 and 7 using these variables Evaluate 'ℓ × 7 Write out the integral for each component Evaluate the integrals one way or another. ...
Chemistry EOC Review
... points, volatility, hardness, electrolytic nature, etc.). You may create a chart if that helps. b. You have two white solids powders in front of you – a covalent solid and an ionic solid. What tests could you do to determine which one was ionic and which one was covalent? (Think in terms of solubili ...
... points, volatility, hardness, electrolytic nature, etc.). You may create a chart if that helps. b. You have two white solids powders in front of you – a covalent solid and an ionic solid. What tests could you do to determine which one was ionic and which one was covalent? (Think in terms of solubili ...
Electrochemistry
... (there is something called the Nernst equation, but that has been removed from the AP curriculum - you will probably come across it at some point) ...
... (there is something called the Nernst equation, but that has been removed from the AP curriculum - you will probably come across it at some point) ...
Midterm Exam
... (b) Find the modal gain coefficient, Γg0, and the transparency current, Jtr. Use the logarithmic approximation for the gain, g = g 0 ln (ηi J J tr ) . The gain is in terms the current density, J. (c) For a Power level of P = 1 W, graphically determine the device length, L, that provides the minimum ...
... (b) Find the modal gain coefficient, Γg0, and the transparency current, Jtr. Use the logarithmic approximation for the gain, g = g 0 ln (ηi J J tr ) . The gain is in terms the current density, J. (c) For a Power level of P = 1 W, graphically determine the device length, L, that provides the minimum ...
Honors Chemistry Final Review
... apart on the _________________ In fact, the further apart, the more ionic! A covalent bond forms from the combination of ______________________, including ___________ It has an electronegativity difference that is ___________ which means that the two combining elements will not be far apart on the p ...
... apart on the _________________ In fact, the further apart, the more ionic! A covalent bond forms from the combination of ______________________, including ___________ It has an electronegativity difference that is ___________ which means that the two combining elements will not be far apart on the p ...
Chemistry EOC Review Name
... 9. Classify the following as having good or poor accuracy and good or poor precision: a. A scientist experimentally determines the speed of light to be 2.98 x 108 m/sec. In a second experiment, she determines the speed to be 2.99 x 10 8 m/sec. b. The actual concentration of a solution is found to be ...
... 9. Classify the following as having good or poor accuracy and good or poor precision: a. A scientist experimentally determines the speed of light to be 2.98 x 108 m/sec. In a second experiment, she determines the speed to be 2.99 x 10 8 m/sec. b. The actual concentration of a solution is found to be ...
ZnO nanodots and nanowires
... As is well known the general solution of this equation must be a linear combination of sines an cosines chosen to satisfy the boundary conditions imposed by the well.Since wave function must vanish at z=0,the solutions must be of the form sin(kz) and since it must also vanish at z=d,we must choose ...
... As is well known the general solution of this equation must be a linear combination of sines an cosines chosen to satisfy the boundary conditions imposed by the well.Since wave function must vanish at z=0,the solutions must be of the form sin(kz) and since it must also vanish at z=d,we must choose ...
MICROSCOPY
... of the image is dependent on the CCD used in the camera. Using a typical 2 Megapixel CCD, an image with 1600 x 1200 pixels is generated. The resolution of the image is dependent on the field of view of the lens used with the camera. ...
... of the image is dependent on the CCD used in the camera. Using a typical 2 Megapixel CCD, an image with 1600 x 1200 pixels is generated. The resolution of the image is dependent on the field of view of the lens used with the camera. ...
PROOF COPY 069543APL
... the notch filter, the coherence length of the source is greater than ⌬1 and fringes are visible everywhere in signal S in the variation range of ⌬2. These results emphasize the fact that the correlation peak of Fig. 3 is due to the interferences between the wave fronts emitted by the luminescent sam ...
... the notch filter, the coherence length of the source is greater than ⌬1 and fringes are visible everywhere in signal S in the variation range of ⌬2. These results emphasize the fact that the correlation peak of Fig. 3 is due to the interferences between the wave fronts emitted by the luminescent sam ...
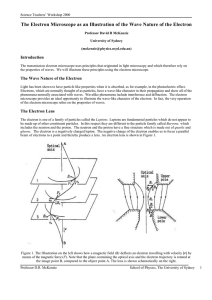
The Electron Microscope as an Illustration of the Wave Nature of the
... Figure 2 The electron paths in the electron microscope operating in imaging mode (left) and diffraction mode (right) In the electron microscope, the source of electrons is a heated filament of tungsten, from which the electrons are emitted or “boiled off”. In advanced forms of the electron microscop ...
... Figure 2 The electron paths in the electron microscope operating in imaging mode (left) and diffraction mode (right) In the electron microscope, the source of electrons is a heated filament of tungsten, from which the electrons are emitted or “boiled off”. In advanced forms of the electron microscop ...
Chemistry I
... low temperatures and low pressures low temperatures and high pressures high temperatures and low pressures high temperatures and high pressures ...
... low temperatures and low pressures low temperatures and high pressures high temperatures and low pressures high temperatures and high pressures ...
Total Notes for chem - Catawba County Schools
... based on these ideas; 1. When elements combine, the outermost electrons; that is, those electrons with levels of energy which will cause them to orbit at the greatest distance from the nucleus, will be the only electrons directly involved with the reaction of these elements to form compounds. 2. The ...
... based on these ideas; 1. When elements combine, the outermost electrons; that is, those electrons with levels of energy which will cause them to orbit at the greatest distance from the nucleus, will be the only electrons directly involved with the reaction of these elements to form compounds. 2. The ...
Cl Cl and
... Too much energy is needed to remove 4 electrons from an atom. Too much energy is needed to insert 4 electrons into an atom in order to overcome the repulsive forces between like charges. 28. Why do elements of groups 6 and 7 form ions of charge –2 and –1 respectively? By gaining electrons they achie ...
... Too much energy is needed to remove 4 electrons from an atom. Too much energy is needed to insert 4 electrons into an atom in order to overcome the repulsive forces between like charges. 28. Why do elements of groups 6 and 7 form ions of charge –2 and –1 respectively? By gaining electrons they achie ...
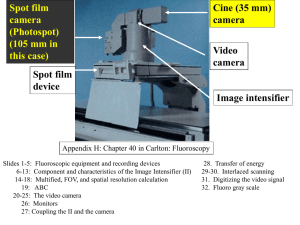
No Slide Title
... The control grid on the camera supplies a constant negative potential to strip electrons from the space charge of the cathode. But the control grid on the monitor is supplied by the video signal. Since the video signals modulates as the encoded image signal, the charge it supplies the control grid i ...
... The control grid on the camera supplies a constant negative potential to strip electrons from the space charge of the cathode. But the control grid on the monitor is supplied by the video signal. Since the video signals modulates as the encoded image signal, the charge it supplies the control grid i ...
Gaseous detection device
The gaseous detection device-GDD is a method and apparatus for the detection of signals in the gaseous environment of an environmental scanning electron microscope (ESEM) and all scanned beam type of instruments that allow a minimum gas pressure for the detector to operate.