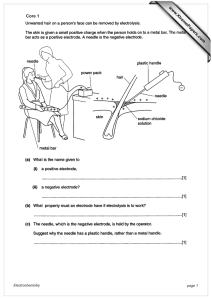
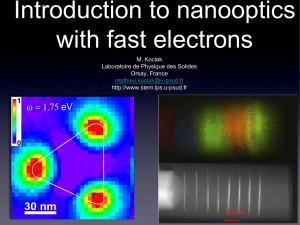
When to use the projection assumption and the weak
... suitable, whereas right/above it is violated. As given by Eq. (11), below the horizontal line sV z ¼ 0:36 the WPOA holds. For the full potential map sampled at 1 Å (green circle), neither PA nor WPOA holds, whereas for the potential map sampled at 3 Å (blue circle) the WPOA is satisfied and the PA is ...
... suitable, whereas right/above it is violated. As given by Eq. (11), below the horizontal line sV z ¼ 0:36 the WPOA holds. For the full potential map sampled at 1 Å (green circle), neither PA nor WPOA holds, whereas for the potential map sampled at 3 Å (blue circle) the WPOA is satisfied and the PA is ...
lesson 5
... LESSON I How do some 5 ^ compounds form .? You have learned about electron shells. Now use this knowledge to understand how atoms link up to form compounds. Not all atoms form compounds. Only atoms that have outer shells that are not full form compounds. The elements of Group 18 have complete outer ...
... LESSON I How do some 5 ^ compounds form .? You have learned about electron shells. Now use this knowledge to understand how atoms link up to form compounds. Not all atoms form compounds. Only atoms that have outer shells that are not full form compounds. The elements of Group 18 have complete outer ...
effective nuclear charge
... for transition metals electrons, may be removed from the sublevel closest to the valence shell Al atom = 1s22s22p63s23p1 Al+3 ion = 1s22s22p6 Fe atom = 1s22s22p63s23p64s23d6 Fe+2 ion = 1s22s22p63s23p63d6 Fe+3 ion = 1s22s22p63s23p63d5 Cu atom = 1s22s22p63s23p64s13d10 Cu+1 ion = 1s22s22p63s23p63d10 ...
... for transition metals electrons, may be removed from the sublevel closest to the valence shell Al atom = 1s22s22p63s23p1 Al+3 ion = 1s22s22p6 Fe atom = 1s22s22p63s23p64s23d6 Fe+2 ion = 1s22s22p63s23p63d6 Fe+3 ion = 1s22s22p63s23p63d5 Cu atom = 1s22s22p63s23p64s13d10 Cu+1 ion = 1s22s22p63s23p63d10 ...
Electrons and Photons
... bonding occurs, the constituent atoms are “free” to wander around. They are in an antibonding state. We could take silicon as an example. When two such free silicon atoms meet, they may bond together. They will do so because the bonding state is at a lower energy than what existed previously. The va ...
... bonding occurs, the constituent atoms are “free” to wander around. They are in an antibonding state. We could take silicon as an example. When two such free silicon atoms meet, they may bond together. They will do so because the bonding state is at a lower energy than what existed previously. The va ...
Extreme Sensitivity in Photoacoustics by Using Optical Cantilever
... The displacement of the cantilever is measured with a compact Michelson interferometer (Fig. 4). The laser beam is directed towards the free end of the cantilever and focused very close to it. Both arms of the interferometer are set to equal and the reference mirror is adjusted in such a way that th ...
... The displacement of the cantilever is measured with a compact Michelson interferometer (Fig. 4). The laser beam is directed towards the free end of the cantilever and focused very close to it. Both arms of the interferometer are set to equal and the reference mirror is adjusted in such a way that th ...
12. CONFOCAL MICROSCOPY • Confocal microscopy can render
... Aberrations, both chromatic and geometrical, lower the resolving power of the confocal microscope. Aberration correction, adaptive optics, is a critical issue especially when operating at very high NA (current research) However, even with an instrument that is capable in principle of producing ...
... Aberrations, both chromatic and geometrical, lower the resolving power of the confocal microscope. Aberration correction, adaptive optics, is a critical issue especially when operating at very high NA (current research) However, even with an instrument that is capable in principle of producing ...
EELS
... ‣One can show that the EELS is a very good approximation of the EMLD ‣EMLDOS is a very concise description of optical excitations ‣The EELS is a map of the plasmons “eigenfunction” (with some marks... ‣The STEM-EELS is a decent plasmonic counterpart of the STM for ...
... ‣One can show that the EELS is a very good approximation of the EMLD ‣EMLDOS is a very concise description of optical excitations ‣The EELS is a map of the plasmons “eigenfunction” (with some marks... ‣The STEM-EELS is a decent plasmonic counterpart of the STM for ...
London_S - Stanford Synchrotron Radiation Lightsource
... the valence shell electrons are excited or ionized. For very high fluence ultra-short pulse optical excitation, non-thermal melting of the lattice can occur on very short time scales (< 200 fs). Thermal motion of the ions is caused by electron-ion coupling. Generally the timescale for this process i ...
... the valence shell electrons are excited or ionized. For very high fluence ultra-short pulse optical excitation, non-thermal melting of the lattice can occur on very short time scales (< 200 fs). Thermal motion of the ions is caused by electron-ion coupling. Generally the timescale for this process i ...
Microscope
... two points as separate and distinct even when they are so close together that they appear as one to the unaided eye. This distance at which two points appear as one is about 0.1 mm at ten inches for the human eye. This distance is called the resolving power and can be expressed as the relationship o ...
... two points as separate and distinct even when they are so close together that they appear as one to the unaided eye. This distance at which two points appear as one is about 0.1 mm at ten inches for the human eye. This distance is called the resolving power and can be expressed as the relationship o ...
Redox
... to zero, and those in an ion must add up to the charge on the ion. Alkali metals have an oxidation number of +1, and alkaline earth metals +2, in their compounds. Fluorine always has an oxidation number of –1 in its compounds. The other halogens have –1 in their compounds except in compounds with ox ...
... to zero, and those in an ion must add up to the charge on the ion. Alkali metals have an oxidation number of +1, and alkaline earth metals +2, in their compounds. Fluorine always has an oxidation number of –1 in its compounds. The other halogens have –1 in their compounds except in compounds with ox ...
Group 2 - Index of
... on band gap, leaves an open range for lasers that are not material dependant. The particular Range that is important is the Mid-IR range (3 to 20 micrometers), where most molecular absorption bands are. QCLs are the only semiconductor laser able to produce mid-IR wavelength beams at or above room te ...
... on band gap, leaves an open range for lasers that are not material dependant. The particular Range that is important is the Mid-IR range (3 to 20 micrometers), where most molecular absorption bands are. QCLs are the only semiconductor laser able to produce mid-IR wavelength beams at or above room te ...
The Photoelectric Effect
... The photoelectric effect was discovered by Heinrich Hertz in the 1880's when he observed that under the right conditions, when light is shined on a metal, electrons are ejected from the surface of the metal. This process was called photoemission. In 1880 light was believed to be completely wavelike ...
... The photoelectric effect was discovered by Heinrich Hertz in the 1880's when he observed that under the right conditions, when light is shined on a metal, electrons are ejected from the surface of the metal. This process was called photoemission. In 1880 light was believed to be completely wavelike ...
Document
... But how does it work? It can only detect one small point. Need to scan the surface need scanning mechanism with ~10 nm resolution It uses piezoelectric elements (expand with voltage) ...
... But how does it work? It can only detect one small point. Need to scan the surface need scanning mechanism with ~10 nm resolution It uses piezoelectric elements (expand with voltage) ...
AOM2017 Abstract Template
... 1. Header An overview of the recent developments in the field of plasmonics using vectorial optical fields as the excitation is presented. Surface plasmons are collective oscillation of free electrons at metal/dielectric interface. As a wave phenomenon, surface plasmons can be focused by using appro ...
... 1. Header An overview of the recent developments in the field of plasmonics using vectorial optical fields as the excitation is presented. Surface plasmons are collective oscillation of free electrons at metal/dielectric interface. As a wave phenomenon, surface plasmons can be focused by using appro ...
IOSR Journal of Applied Physics (IOSR-JAP)
... ) , Neon (Ne), Fluorine( F2) and chlorine (CL2). These gas were heated at temperature in the range ( 300 -337ºk.) The spectra of these gas were displayed by USB2000 spectrometer. The result shows appreciable change in the spectral intensity and line width. These changes was shown to be explained the ...
... ) , Neon (Ne), Fluorine( F2) and chlorine (CL2). These gas were heated at temperature in the range ( 300 -337ºk.) The spectra of these gas were displayed by USB2000 spectrometer. The result shows appreciable change in the spectral intensity and line width. These changes was shown to be explained the ...
Photons and Matter Waves
... a metal surface, a stream of electrons emerges from the metal. The interference filter is then replaced with one transmitting at 300 nm and the lamp adjusted so that the intensity of the light striking the surface is the same as it was for the 400-nm light. With the 300-nm light, 1) more electrons a ...
... a metal surface, a stream of electrons emerges from the metal. The interference filter is then replaced with one transmitting at 300 nm and the lamp adjusted so that the intensity of the light striking the surface is the same as it was for the 400-nm light. With the 300-nm light, 1) more electrons a ...
AP Chemistry Unit 3 Test Review Topics Covered: Gases Liquids
... 17) The best explanation for the lower pressure in container 4 is that SO2 molecules (A) have a larger average speed than the other three gases (B) occupy a larger portion of the container volume than the other three gases (C) have stronger intermolecular attractions than the other three gases (D) c ...
... 17) The best explanation for the lower pressure in container 4 is that SO2 molecules (A) have a larger average speed than the other three gases (B) occupy a larger portion of the container volume than the other three gases (C) have stronger intermolecular attractions than the other three gases (D) c ...
CHEM 20 FINAL EXAM: STUDY HEADINGS Jan 2012
... Which of the following is an example of combustion? a) sodium metal + chlorine gas produces sodium chloride solid b) propane + oxygen produces carbon dioxide and water c) sulfuric acid decomposes into hydrogen gas, oxygen gas and sulfur d) solid lead metal reacts with a silver nitrate solution to pr ...
... Which of the following is an example of combustion? a) sodium metal + chlorine gas produces sodium chloride solid b) propane + oxygen produces carbon dioxide and water c) sulfuric acid decomposes into hydrogen gas, oxygen gas and sulfur d) solid lead metal reacts with a silver nitrate solution to pr ...
Gaseous detection device
The gaseous detection device-GDD is a method and apparatus for the detection of signals in the gaseous environment of an environmental scanning electron microscope (ESEM) and all scanned beam type of instruments that allow a minimum gas pressure for the detector to operate.