12. confocal microscopy.
... Note that confocal microscopy is intrinsically an intensity-based method. Therefore, we anticipate low contrast when operating in reflective mode, as the refractive index variation within tissues is not very high. Further, the residual out of focus light that makes it to the detector has the effect ...
... Note that confocal microscopy is intrinsically an intensity-based method. Therefore, we anticipate low contrast when operating in reflective mode, as the refractive index variation within tissues is not very high. Further, the residual out of focus light that makes it to the detector has the effect ...
4.1 PPT- Atomic Theory and Bonding
... Some metals are MULTIVALENT and can lose a varying number of electrons. For example, iron, Fe, loses either two (Fe2+) or three (Fe3+) electrons ...
... Some metals are MULTIVALENT and can lose a varying number of electrons. For example, iron, Fe, loses either two (Fe2+) or three (Fe3+) electrons ...
OPTION.physics new
... organism to maintain temperature, fluid flow rate and similar other biological function. The two basic characteristics of control system are: (i) In this process input gives indication of both the controlled variable and its desired value expressed in the same unit. (ii) The output signal represents ...
... organism to maintain temperature, fluid flow rate and similar other biological function. The two basic characteristics of control system are: (i) In this process input gives indication of both the controlled variable and its desired value expressed in the same unit. (ii) The output signal represents ...
HIGHER TIER CHEMISTRY MINI-MOCK UNIT 2
... a layer of gel containing ions in solution. The positive electrode is connected by a patch to the skin.The negative electrode is connected to the hair. Electricity flows through the gel and causes electrolysis of the body fluid around the hair follicle. (a) Metal wires conduct electricity to the ele ...
... a layer of gel containing ions in solution. The positive electrode is connected by a patch to the skin.The negative electrode is connected to the hair. Electricity flows through the gel and causes electrolysis of the body fluid around the hair follicle. (a) Metal wires conduct electricity to the ele ...
chemistry
... If you wish to change an answer, erase your first penciled circle and then circle with pencil the number of the answer you want. After you have completed the examination and you have decided that all of the circled answers represent your best judgment, signal a proctor and turn in all examination ma ...
... If you wish to change an answer, erase your first penciled circle and then circle with pencil the number of the answer you want. After you have completed the examination and you have decided that all of the circled answers represent your best judgment, signal a proctor and turn in all examination ma ...
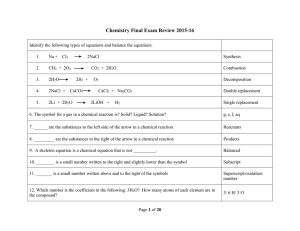
Type Of Chemical Reaction
... the particles in a gas are relatively far apart, the particles in a gas move independently of each other. ...
... the particles in a gas are relatively far apart, the particles in a gas move independently of each other. ...
Electrodynamics of Solids
... this then implies that A has only transverse components, perpendicular to the wavevector q. Of course one cannot do justice to all the various interesting effects which arise in the different forms of condensed matter – certain selections have to be made, this being influenced by our prejudices. We ...
... this then implies that A has only transverse components, perpendicular to the wavevector q. Of course one cannot do justice to all the various interesting effects which arise in the different forms of condensed matter – certain selections have to be made, this being influenced by our prejudices. We ...
Photoacoustics Spectroscopy
... acoustic mode Pj ( is the complex conjugate of pj), and the integral is over the volume of the cell. Qj accounts for the mode damping and avoid the physically unreasonable situation of as Equation 12 may be further simplified for the case H being given by equations 2 and 3. In this case ...
... acoustic mode Pj ( is the complex conjugate of pj), and the integral is over the volume of the cell. Qj accounts for the mode damping and avoid the physically unreasonable situation of as Equation 12 may be further simplified for the case H being given by equations 2 and 3. In this case ...
AP Chemistry Second Semester Notes
... side of stair step and H N3- > O2- > F- > Ne > Na+ > Mg2+ > Al3+ 1. opposite properties to metals 4. ionization energy 2. form molecules in addition to ionic compounds a. E to remove electron from a gaseous atom 3. large positive ionization energy 1. X(g) X+(g) + 1e4. large negative electron affi ...
... side of stair step and H N3- > O2- > F- > Ne > Na+ > Mg2+ > Al3+ 1. opposite properties to metals 4. ionization energy 2. form molecules in addition to ionic compounds a. E to remove electron from a gaseous atom 3. large positive ionization energy 1. X(g) X+(g) + 1e4. large negative electron affi ...
Chapter 5 of Zumdahl
... explains why ideal gases behave the way they do. Assumptions that simplify the theory, but don’t work in real gases. 1 The particles are so small we can ignore their volume. The particles are in constant motion and their collisions cause pressure. ...
... explains why ideal gases behave the way they do. Assumptions that simplify the theory, but don’t work in real gases. 1 The particles are so small we can ignore their volume. The particles are in constant motion and their collisions cause pressure. ...
Chapter V: Electrons in Atoms
... • Principal energy levels contain energy ________. • Energy level _ = _ sublevel; _____; _____; etc. • __________ of energy sublevels in a principal energy level ________ as n _____________. ...
... • Principal energy levels contain energy ________. • Energy level _ = _ sublevel; _____; _____; etc. • __________ of energy sublevels in a principal energy level ________ as n _____________. ...
Spectroscopic Study of Argon DC Glow Discharge
... from the NG and PC of the dc glow discharge in argon gas has been performed. The line intensities of different lines have been measured as a function of gas pressure and the discharge current. The intensity of the lines in the NG is about four times that in the PC. The intensity of the lines has bee ...
... from the NG and PC of the dc glow discharge in argon gas has been performed. The line intensities of different lines have been measured as a function of gas pressure and the discharge current. The intensity of the lines in the NG is about four times that in the PC. The intensity of the lines has bee ...
System International Base Units
... When doing electron configurations for ions, subtract the charge of the ion to its atomic number to get the number of electrons needed for the electron configuration. You should notice the electron configuration of group A ions are always the same as their nearest noble gas o Example: Write the elec ...
... When doing electron configurations for ions, subtract the charge of the ion to its atomic number to get the number of electrons needed for the electron configuration. You should notice the electron configuration of group A ions are always the same as their nearest noble gas o Example: Write the elec ...
Einstein`s paper is a “bold, not to say reckless, hypothesis…which
... The stopping voltage Vstop (the maximum kinetic energy of electrons) increases linearly with frequency. Below a certain frequency fo, no electrons are emitted, even for intense light! This makes no sense classically. Increasing the electric field should have an effect. Lecture 7, p 12 ...
... The stopping voltage Vstop (the maximum kinetic energy of electrons) increases linearly with frequency. Below a certain frequency fo, no electrons are emitted, even for intense light! This makes no sense classically. Increasing the electric field should have an effect. Lecture 7, p 12 ...
System International Base Units
... When doing electron configurations for ions, subtract the charge of the ion to its atomic number to get the number of electrons needed for the electron configuration. You should notice the electron configuration of group A ions are always the same as their nearest noble gas o Example: Write the elec ...
... When doing electron configurations for ions, subtract the charge of the ion to its atomic number to get the number of electrons needed for the electron configuration. You should notice the electron configuration of group A ions are always the same as their nearest noble gas o Example: Write the elec ...
Artificial Photosynthesis: A Workshop in Solar Cell Design
... substances, i.e. fuels, are produced in these reactions from very simple, low‐energy starting materials powered by a readily available and abundant form of energy in sunlight. Solar cells mimic the light absorption and electron flow of photosynthesis to produce electricity. The basic ...
... substances, i.e. fuels, are produced in these reactions from very simple, low‐energy starting materials powered by a readily available and abundant form of energy in sunlight. Solar cells mimic the light absorption and electron flow of photosynthesis to produce electricity. The basic ...
Color Distribution of Light Balls in Hessdalen Lights Pheomenon
... large distance (on the order of 50–100 m) from the large white-colored nucleus tend to be green-colored, while the small balls that appear to be very close (distance on the order of 2–5 m) to a cluster nucleus tend to be white- (high intensity) or red- (high intensity) and blue- (low-intensity) colo ...
... large distance (on the order of 50–100 m) from the large white-colored nucleus tend to be green-colored, while the small balls that appear to be very close (distance on the order of 2–5 m) to a cluster nucleus tend to be white- (high intensity) or red- (high intensity) and blue- (low-intensity) colo ...
Chemistry- The Gas Phase
... At constant P and T, the V of a gas is directly proportional to the # of moles (n) of the gas. V/n = constant ...
... At constant P and T, the V of a gas is directly proportional to the # of moles (n) of the gas. V/n = constant ...
Chemistry Final Exam Review 2006-2007
... Which of the following elements has the same number of valence electrons as the element sodium? a. Ar b. Cs c. Ca d. Mg Which of the following elements has the same number of valence electrons as the element selenium? a. Fe b. K c. P d. O Which of the following elements will have similar physical an ...
... Which of the following elements has the same number of valence electrons as the element sodium? a. Ar b. Cs c. Ca d. Mg Which of the following elements has the same number of valence electrons as the element selenium? a. Fe b. K c. P d. O Which of the following elements will have similar physical an ...
3.7 Dielectrics and Optics 3.7.1 Basics
... If we now consider the refracted beam, we know that it travels under an angle β, has the same frequency as the incident beam, but a wavelength λd and a velocity c that is different from λi and c0. Moreover, we must expect that it is damped or attenuated, i.e. that its amplitude decreases as a functi ...
... If we now consider the refracted beam, we know that it travels under an angle β, has the same frequency as the incident beam, but a wavelength λd and a velocity c that is different from λi and c0. Moreover, we must expect that it is damped or attenuated, i.e. that its amplitude decreases as a functi ...
Gaseous detection device
The gaseous detection device-GDD is a method and apparatus for the detection of signals in the gaseous environment of an environmental scanning electron microscope (ESEM) and all scanned beam type of instruments that allow a minimum gas pressure for the detector to operate.