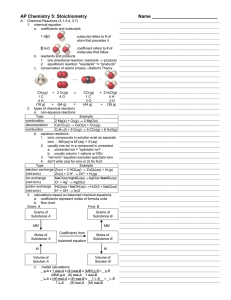
Mole Concept and Stoichiometry
... contains an unimaginable number of them. Therefore, counting the number of atoms or molecules in a sample is impossible. The multiple interpretations of the mole allow us to bridge the gap between the submicroscopic world of atoms and molecules and the macroscopic world that we can observe. To deter ...
... contains an unimaginable number of them. Therefore, counting the number of atoms or molecules in a sample is impossible. The multiple interpretations of the mole allow us to bridge the gap between the submicroscopic world of atoms and molecules and the macroscopic world that we can observe. To deter ...
Questions
... excess ethanoyl chloride in the presence of the catalyst anhydrous aluminium chloride. ...
... excess ethanoyl chloride in the presence of the catalyst anhydrous aluminium chloride. ...
Part One: Ions in Aqueous Solution A. Electrolytes and Non
... Net ionic equation = complete ionic equation after “spectator ions” have been canceled out. 2H+(aq) + Mg(OH)2(s) → Mg+ (aq) + 2H2O [This is the essence of the reaction.] ...
... Net ionic equation = complete ionic equation after “spectator ions” have been canceled out. 2H+(aq) + Mg(OH)2(s) → Mg+ (aq) + 2H2O [This is the essence of the reaction.] ...
LESSON ASSIGNMENT LESSON 2 Elements of Chemical Change
... balance chemical equations. You will, however, be using and/or seeing the effects of chemical reactions on a daily basis. Chemical reactions are frequently used to explain various concepts of pharmacology and physiology. Consider drugs. All drugs are chemicals and any pharmacological reference you c ...
... balance chemical equations. You will, however, be using and/or seeing the effects of chemical reactions on a daily basis. Chemical reactions are frequently used to explain various concepts of pharmacology and physiology. Consider drugs. All drugs are chemicals and any pharmacological reference you c ...
Covalent Bonding - whitburnscience
... Let’s look at Oxygen. This has a charge of 2- and so is written as O2- so if this reacts with Sodium which is 1+, how many Sodium do we need? That’s right two. ...
... Let’s look at Oxygen. This has a charge of 2- and so is written as O2- so if this reacts with Sodium which is 1+, how many Sodium do we need? That’s right two. ...
Steps for Substitution - Brookwood High School
... 3. If it isn’t, look to see what you could multiply by to eliminate a variable. 4. Rewrite the equation you need to multiply and multiply the whole equation by that number. 5. Rewrite the other equation under it. 6. Add the “columns.” 7. Solve for that variable. 8. Substitute that answer into one of ...
... 3. If it isn’t, look to see what you could multiply by to eliminate a variable. 4. Rewrite the equation you need to multiply and multiply the whole equation by that number. 5. Rewrite the other equation under it. 6. Add the “columns.” 7. Solve for that variable. 8. Substitute that answer into one of ...
Conics - Parabolas
... a plane, the plane will pass through the base of the cone, but will only pass through one of the cones. Any of these conics can be graphed on a coordinate plane. The graph of any conic on an x-y coordinate plane can be described by an equation in the form: Ax2 + Bxy + Cy2 + Dx + Ey + F = 0 This equa ...
... a plane, the plane will pass through the base of the cone, but will only pass through one of the cones. Any of these conics can be graphed on a coordinate plane. The graph of any conic on an x-y coordinate plane can be described by an equation in the form: Ax2 + Bxy + Cy2 + Dx + Ey + F = 0 This equa ...
Pass Assured`s Pharmacy Technician Training Program
... A milliequivalent is equal to the number of univalent counter ions (H+ or OH) which will be needed to react with one molecule of the substance When an atom has a valence of one ( e.g. Na+, K+, CI, etc.) a mEq of that ion is equal to the atomic weight of the atom in milligrams. For example, one mEq o ...
... A milliequivalent is equal to the number of univalent counter ions (H+ or OH) which will be needed to react with one molecule of the substance When an atom has a valence of one ( e.g. Na+, K+, CI, etc.) a mEq of that ion is equal to the atomic weight of the atom in milligrams. For example, one mEq o ...
1 The Mole 6.02 X 10 23
... Other Names Related to Molar Mass • Molecular Mass/Molecular Weight: If you have a single molecule, mass is measured in amu’s instead of grams. But, the molecular mass/weight is the same numerical value as 1 mole of molecules. Only the units are different. (This is the beauty of Avogadro’s Number!) ...
... Other Names Related to Molar Mass • Molecular Mass/Molecular Weight: If you have a single molecule, mass is measured in amu’s instead of grams. But, the molecular mass/weight is the same numerical value as 1 mole of molecules. Only the units are different. (This is the beauty of Avogadro’s Number!) ...
Knowing the slope of a line, m, and the y
... Aim #20: How do we graph lines in slope-intercept form? Homework: Text pg 115 (1-9, 12-13, 16-21), pg 120 (2-20 even #s only) Do Now: Find the intercepts of the graph below. ...
... Aim #20: How do we graph lines in slope-intercept form? Homework: Text pg 115 (1-9, 12-13, 16-21), pg 120 (2-20 even #s only) Do Now: Find the intercepts of the graph below. ...