STORAGE AND FLOW OF FLUIDS AND ELECTRICITY
... increase in the second. Since the quantity of oil in a container sets up the pressure due to the action of gravity, the pressure difference between the tanks will go down, which will make the current of oil through the pipe decrease, and this will make the levels of oil in the tanks change more slow ...
... increase in the second. Since the quantity of oil in a container sets up the pressure due to the action of gravity, the pressure difference between the tanks will go down, which will make the current of oil through the pipe decrease, and this will make the levels of oil in the tanks change more slow ...
Chapter 4
... hand, certain acids, such as acetic acid (CH3COOH), which gives vinegar its tart flavor, do not ionize completely and are weak electrolytes. We represent the ionization of acetic acid as CH3COOH(aq) Δ CH3COO2 (aq) 1 H1 (aq) where CH3COO2 is called the acetate ion. We use the term ionization to descr ...
... hand, certain acids, such as acetic acid (CH3COOH), which gives vinegar its tart flavor, do not ionize completely and are weak electrolytes. We represent the ionization of acetic acid as CH3COOH(aq) Δ CH3COO2 (aq) 1 H1 (aq) where CH3COO2 is called the acetate ion. We use the term ionization to descr ...
Introduction to Electronics Laboratory Manual
... Therefore at reverse bias, the current through the diode strongly increases with temperature. Even at forward bias, the current through the diode will increase with temperature, for the same applied voltage, as the variation of the saturation current will be stronger than the one in the opposite dir ...
... Therefore at reverse bias, the current through the diode strongly increases with temperature. Even at forward bias, the current through the diode will increase with temperature, for the same applied voltage, as the variation of the saturation current will be stronger than the one in the opposite dir ...
Chemical Reactions and Solution Stoichiometry
... oxygen atoms are near the Na+ cations and the hydrogen atoms are near the Cl− anions. This is due to the polar nature of water, a result of uneven electron distribution in water molecules. ( Flashforward to Section 8.6 Molecular Polarity) Water is a neutral compound, but the electrons in the covale ...
... oxygen atoms are near the Na+ cations and the hydrogen atoms are near the Cl− anions. This is due to the polar nature of water, a result of uneven electron distribution in water molecules. ( Flashforward to Section 8.6 Molecular Polarity) Water is a neutral compound, but the electrons in the covale ...
Grade 11 review answers
... 21) Identify a set of test solutions and a correct sequence for adding them to an unknown solution to test for the presence of one or more of the following metal ions: Mercury (2+), Silver, and Barium Use table 1 at the end of the review. 1) Acetate ion (as aqueous Sodium acetate) will precipitate A ...
... 21) Identify a set of test solutions and a correct sequence for adding them to an unknown solution to test for the presence of one or more of the following metal ions: Mercury (2+), Silver, and Barium Use table 1 at the end of the review. 1) Acetate ion (as aqueous Sodium acetate) will precipitate A ...
Dyadic Green`s functions and guided surface waves for a surface
... and, therefore, if ⬙ ⬍ 0 共intraband conductivity dominates兲, the mode is a surface wave on the proper sheet, whereas, if ⬙ ⬎ 0 共interband conductivity dominates兲, the mode is on the improper sheet. In summary, for isolated grapheme, a proper TE surface wave exists if ⬙ ⬎ 0, resulting in the radia ...
... and, therefore, if ⬙ ⬍ 0 共intraband conductivity dominates兲, the mode is a surface wave on the proper sheet, whereas, if ⬙ ⬎ 0 共interband conductivity dominates兲, the mode is on the improper sheet. In summary, for isolated grapheme, a proper TE surface wave exists if ⬙ ⬎ 0, resulting in the radia ...
Halometallate ionic liquids – revisited
... Ionic liquids with chlorometallate anions may not have been the first ionic liquids, however, it was their development that lead to the recognition that ionic liquids are a distinct, and useful, class of (functional) materials. While much of the phenomenal interest and attention over the past two de ...
... Ionic liquids with chlorometallate anions may not have been the first ionic liquids, however, it was their development that lead to the recognition that ionic liquids are a distinct, and useful, class of (functional) materials. While much of the phenomenal interest and attention over the past two de ...
UNITS OF CONCENTRATION
... Parts per million concentrations are essentially mass ratios (solute to solution) x a million (106). In this sense, they are similar to wt %, which could be thought of as parts per hundred (although nobody uses this term). Since 106 milligrams = 1 kg, 1 mg/kg is equivalent to 1 ppm. Similarly, 1 g/ ...
... Parts per million concentrations are essentially mass ratios (solute to solution) x a million (106). In this sense, they are similar to wt %, which could be thought of as parts per hundred (although nobody uses this term). Since 106 milligrams = 1 kg, 1 mg/kg is equivalent to 1 ppm. Similarly, 1 g/ ...
Transferred Electron Devices (TEDs)
... bulk semiconductors by transferring electrons from high-mobility energy bands to low-mobility energy bands was taken a step further by Hilsum in 1962 [3]. Hilsum carefully calculated the transferred electron effect in several III-V compounds and was the first to use the terms transferred electron am ...
... bulk semiconductors by transferring electrons from high-mobility energy bands to low-mobility energy bands was taken a step further by Hilsum in 1962 [3]. Hilsum carefully calculated the transferred electron effect in several III-V compounds and was the first to use the terms transferred electron am ...
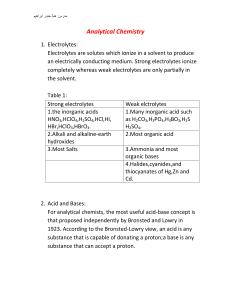
Analytical Chemistry
... information thus, C2H6O is both the empirical and the chemical formula for the chemically ferent ethanol , C2H5OH, and dimethyl ether CH3OCH3 . 5. The mole: It is gram formula weight per formula weight (M.wt.,which is the summation of the atomic weights in grams for all of the atoms in the atoms in ...
... information thus, C2H6O is both the empirical and the chemical formula for the chemically ferent ethanol , C2H5OH, and dimethyl ether CH3OCH3 . 5. The mole: It is gram formula weight per formula weight (M.wt.,which is the summation of the atomic weights in grams for all of the atoms in the atoms in ...
analytical chemistry - Львівський національний медичний
... Group reactions use for selection from complex (complicated) mixes some substances. Substances with definite properties are united in special analytical groups. This reactions use for: a) detection the present analytical group; b) selection this analytical group from another during systematic path ( ...
... Group reactions use for selection from complex (complicated) mixes some substances. Substances with definite properties are united in special analytical groups. This reactions use for: a) detection the present analytical group; b) selection this analytical group from another during systematic path ( ...
Unit - II Electrochemistry
... 5. Electrolyte: Water soluble substance forming ions in solution and conducts electric current 6. Anode compartment: Compartment of the cell in which the oxidation half reaction occurs. It contains the anode 7. Cathode compartment: Compartment of the cell in which the reduction half reaction occurs. ...
... 5. Electrolyte: Water soluble substance forming ions in solution and conducts electric current 6. Anode compartment: Compartment of the cell in which the oxidation half reaction occurs. It contains the anode 7. Cathode compartment: Compartment of the cell in which the reduction half reaction occurs. ...
Outline of Content - College of Micronesia
... Isolation of electric circuits; Tagging; Testing procedures; Basic fault finding procedure; and Re-establishing circuits in accordance with established procedure and safety requirements is described. Possible causes of electrical shock, prevention, and actions to be taken are identified. A ...
... Isolation of electric circuits; Tagging; Testing procedures; Basic fault finding procedure; and Re-establishing circuits in accordance with established procedure and safety requirements is described. Possible causes of electrical shock, prevention, and actions to be taken are identified. A ...
Influence of Ionic Mobile Phase Additives with Low Charge
... distance from surface. In this model, two layers are under dynamic equilibrium, the primary layer is due to the adsorption of the ion pair reagent and the second layer is diffuse and contains the ion pair reagent counter ion. In this model, analyte retention is directed by the free energy of adsorpt ...
... distance from surface. In this model, two layers are under dynamic equilibrium, the primary layer is due to the adsorption of the ion pair reagent and the second layer is diffuse and contains the ion pair reagent counter ion. In this model, analyte retention is directed by the free energy of adsorpt ...
Nanofluidic circuitry
Nanofluidic circuitry is a nanotechnology aiming for control of fluids in nanometer scale. Due to the effect of an electrical double layer within the fluid channel, the behavior of nanofluid is observed to be significantly different compared with its microfluidic counterparts. Its typical characteristic dimensions fall within the range of 1–100 nm. At least one dimension of the structure is in nanoscopic scale. Phenomena of fluids in nano-scale structure are discovered to be of different properties in electrochemistry and fluid dynamics.