University Physics 227N/232N Current and Ohm`s Law, Resistors
... § Electrons often collide with ions (nuclei) in the metal’s crystal structure § They usually lose energy this way § This limits how easily the electrons “flow” through the material ...
... § Electrons often collide with ions (nuclei) in the metal’s crystal structure § They usually lose energy this way § This limits how easily the electrons “flow” through the material ...
Resistance in the Electrical System
... 18. What rules must a technician follow for series circuit? 1. Total resistance = sum of individual resistors 2. Current through each resistor is the same as the current through the whole circuit 3. Sum of each of the voltages across the resistors equal the voltage of the battery ...
... 18. What rules must a technician follow for series circuit? 1. Total resistance = sum of individual resistors 2. Current through each resistor is the same as the current through the whole circuit 3. Sum of each of the voltages across the resistors equal the voltage of the battery ...
1aUnit Two Handouts - Dunmore High School
... If yes, write it as ions. Example: NaOH becomes Na+ + OHIf no, do not write it as ions. Example: Fe(OH)3 stays Fe(OH)3 (Note: Most hydroxides not listed above are weak or nonelectrolytes because they are insoluble in water—always check this out when writing net ionic equations.) ...
... If yes, write it as ions. Example: NaOH becomes Na+ + OHIf no, do not write it as ions. Example: Fe(OH)3 stays Fe(OH)3 (Note: Most hydroxides not listed above are weak or nonelectrolytes because they are insoluble in water—always check this out when writing net ionic equations.) ...
A d f T d A d f T d Agenda for Today
... The nonuniform distribution of surface charges along a wire creates a net electric field inside the wire that points from the more positive end toward the more negative end of the wire. ...
... The nonuniform distribution of surface charges along a wire creates a net electric field inside the wire that points from the more positive end toward the more negative end of the wire. ...
Powerpoints - Holy Cross Collegiate
... • In most reactions, one of the reactants may run out before the others. There will usually be one or more of the reactants left over without getting a chance to completely react. ...
... • In most reactions, one of the reactants may run out before the others. There will usually be one or more of the reactants left over without getting a chance to completely react. ...
Sc 9 Electricity LAB BOOKLET
... Solution conductivity is a powerful tool for studying the environment. Electrical conductivity (EC) increases with the number of ions dissolved in water. This means that conductivity readings can be used as indicators of water quality and the composition of the surrounding soil. Higher EC values in ...
... Solution conductivity is a powerful tool for studying the environment. Electrical conductivity (EC) increases with the number of ions dissolved in water. This means that conductivity readings can be used as indicators of water quality and the composition of the surrounding soil. Higher EC values in ...
Axial ratio improvement in aperture antennas using high
... the E-plane radiation pattern in Fig. 3 is quite broad. TE waves, on the other hand, cannot propagate at grazing angles to a metal surface because their transverse electric field is shorted by the conducting surface. The H-plane is therefore much narrower. This is the expected radiation pattern for ...
... the E-plane radiation pattern in Fig. 3 is quite broad. TE waves, on the other hand, cannot propagate at grazing angles to a metal surface because their transverse electric field is shorted by the conducting surface. The H-plane is therefore much narrower. This is the expected radiation pattern for ...
Get Solutions - Iqraa group of institutes
... that of R2 by 10 kJ mol-1. If k1 and k2 are rate constants for reactions R1 and R2 respectively at 300 K,/then ln(k2/k1) is equal to : (R = 8.814 J mol-1 K-1) ...
... that of R2 by 10 kJ mol-1. If k1 and k2 are rate constants for reactions R1 and R2 respectively at 300 K,/then ln(k2/k1) is equal to : (R = 8.814 J mol-1 K-1) ...
Mathematical Modeling of the Formation of Calcareous
... the influence of physics and chemistry of seawater, cathodic protection, and surface preparation on the formation of calcareous deposits through electrochemical experiments in natural seawater 512 and in artificial seawater. 13-~4 However, there are very few papers regarding mathematical modeling of ...
... the influence of physics and chemistry of seawater, cathodic protection, and surface preparation on the formation of calcareous deposits through electrochemical experiments in natural seawater 512 and in artificial seawater. 13-~4 However, there are very few papers regarding mathematical modeling of ...
PowerPoint Presentation - Lecture 1 Electric Charge*
... to be enclosed by a coaxial, thin walled, nonconducting cylindrical shell of radius 1.8 cm. The shell is to have positive charge on its outside surface with a surface charge density that makes the net external electric field zero. Calculate the surface charge density. ...
... to be enclosed by a coaxial, thin walled, nonconducting cylindrical shell of radius 1.8 cm. The shell is to have positive charge on its outside surface with a surface charge density that makes the net external electric field zero. Calculate the surface charge density. ...
Chemistry II Exams and Keys 2013 Season
... 5. Which of the following compounds has the highest nitrogen content by mass? A. ammonium nitrate, NH4NO3 B. aluminum nitrite, Al(NO2)3 C. sodium azide, NaN3 D. potassium nitrate, KNO3 E. lithium nitride, Li3N 6. Cadmium metal is used in electroplating industry. It is an extremely toxic element. An ...
... 5. Which of the following compounds has the highest nitrogen content by mass? A. ammonium nitrate, NH4NO3 B. aluminum nitrite, Al(NO2)3 C. sodium azide, NaN3 D. potassium nitrate, KNO3 E. lithium nitride, Li3N 6. Cadmium metal is used in electroplating industry. It is an extremely toxic element. An ...
Electric Potential
... When a capacitor is subjected to an electric potential across its terminals, the electrons will move accordingly. When charging a capacitor, it will initially behave much like a wire, allowing very high current flow with a very small potential drop. When a capacitor nears the electric potential of t ...
... When a capacitor is subjected to an electric potential across its terminals, the electrons will move accordingly. When charging a capacitor, it will initially behave much like a wire, allowing very high current flow with a very small potential drop. When a capacitor nears the electric potential of t ...
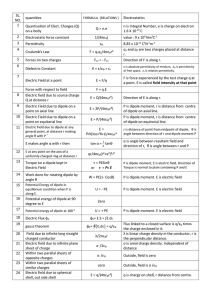
SL. NO. quantities FORMULA (RELATIONS ) Electrostatics 1
... Potential Energy is that of the system containing Q and q. V = (v2 -v1) ...
... Potential Energy is that of the system containing Q and q. V = (v2 -v1) ...
Nanofluidic circuitry
Nanofluidic circuitry is a nanotechnology aiming for control of fluids in nanometer scale. Due to the effect of an electrical double layer within the fluid channel, the behavior of nanofluid is observed to be significantly different compared with its microfluidic counterparts. Its typical characteristic dimensions fall within the range of 1–100 nm. At least one dimension of the structure is in nanoscopic scale. Phenomena of fluids in nano-scale structure are discovered to be of different properties in electrochemistry and fluid dynamics.