Solutions for Chapter 8 End-of-Chapter Problems
... Solutions for Chapter 8 End-of-Chapter Problems Problem 8.1. (a) Ice cream melting will decrease the organization of the system. Rather than being in identifiable scoops of ice cream perched securely on a cone, there now will be liquid ice cream dripping on the outside of the cone, onto your hands, ...
... Solutions for Chapter 8 End-of-Chapter Problems Problem 8.1. (a) Ice cream melting will decrease the organization of the system. Rather than being in identifiable scoops of ice cream perched securely on a cone, there now will be liquid ice cream dripping on the outside of the cone, onto your hands, ...
Stoichiometry: Calculations with Chemical Formulas and
... Molar Mass • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... Molar Mass • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
Computational Studies on Conformations and Properties of Peptide
... shows that the same types of monovalent metal ion (Na+ and Rb+) coordinated complexes have similar spectra, but the spectra of divalent metal ion (Mg 2+) coordinated complexes are quite different. The conformational distributions of all the complexes at various temperatures were investigated, which ...
... shows that the same types of monovalent metal ion (Na+ and Rb+) coordinated complexes have similar spectra, but the spectra of divalent metal ion (Mg 2+) coordinated complexes are quite different. The conformational distributions of all the complexes at various temperatures were investigated, which ...
AP Ch 3 Stoichiometry
... 2 Al + 3 Cl2 2 AlCl3 • We found that chlorine is the limiting reactant, and 43.8 g of aluminum chloride are produced. 35.0 g Cl2 1 mol Cl2 2 mol Al 71 g Cl2 ...
... 2 Al + 3 Cl2 2 AlCl3 • We found that chlorine is the limiting reactant, and 43.8 g of aluminum chloride are produced. 35.0 g Cl2 1 mol Cl2 2 mol Al 71 g Cl2 ...
Evolutionary Patterns in the Sequence and Structure of
... triplets of bases on their ‘anticodon’ arms recognize complementary ‘codon’ sequences in messenger RNA. These and many other molecular interactions define the identities and functions of these tRNA adaptors and establish a genetic code that translates nucleic acid into protein information in the cel ...
... triplets of bases on their ‘anticodon’ arms recognize complementary ‘codon’ sequences in messenger RNA. These and many other molecular interactions define the identities and functions of these tRNA adaptors and establish a genetic code that translates nucleic acid into protein information in the cel ...
The Cutting Edge of Affinity Electrophoresis Technology
... of 10 pg/mL proved that this method is highly sensitive. One advantage of CAE is that affinity probes can be prepared for various molecules ranging from macromolecules (such as antibodies) to low-molecular-weight compounds. Another advantage is that CAE separations can be completed with short migrat ...
... of 10 pg/mL proved that this method is highly sensitive. One advantage of CAE is that affinity probes can be prepared for various molecules ranging from macromolecules (such as antibodies) to low-molecular-weight compounds. Another advantage is that CAE separations can be completed with short migrat ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... Analyze: We are given several chemical formulas and asked to classify each substance as a strong electrolyte, weak electrolyte, or nonelectrolyte. Plan: The approach we take is outlined in Table 4.3. We can predict whether a substance is ionic or molecular, based on its composition. As we saw in Sec ...
... Analyze: We are given several chemical formulas and asked to classify each substance as a strong electrolyte, weak electrolyte, or nonelectrolyte. Plan: The approach we take is outlined in Table 4.3. We can predict whether a substance is ionic or molecular, based on its composition. As we saw in Sec ...
Moles Class Packet Unit 2
... 12. If 3.87x1025 molecules of water are used, how many liters of hydrogen gas form at STP? ...
... 12. If 3.87x1025 molecules of water are used, how many liters of hydrogen gas form at STP? ...
Chapter 3 Sem 2 2013-14
... Think of the mole as a counting unit for a collection of: 6.02 x 1023 things---unlike all other counting numbers it is also linked to masses given in the ...
... Think of the mole as a counting unit for a collection of: 6.02 x 1023 things---unlike all other counting numbers it is also linked to masses given in the ...
Covalent Bonding and Nomenclature
... Binary Molecular Nomenclature Compounds formed when atoms covalently bond are called molecular compounds. Binary molecular compounds are generally composed of two nonmetallic elements. When two nonmetallic elements combine, they often do so in more than one way. For example carbon can combine with ...
... Binary Molecular Nomenclature Compounds formed when atoms covalently bond are called molecular compounds. Binary molecular compounds are generally composed of two nonmetallic elements. When two nonmetallic elements combine, they often do so in more than one way. For example carbon can combine with ...
Chapter
... Types of Formula Structural Formula • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess a ...
... Types of Formula Structural Formula • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess a ...
Chapter
... Types of Formula Structural Formula • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess a ...
... Types of Formula Structural Formula • Structural Formula describe the kinds of elements found in the compound, the numbers of their atoms, order of atom attachment, and the kind of attachment they do not directly describe the 3-dimensional shape, but an experienced chemist can make a good guess a ...
On-surface derivatisation of aromatic molecules
... molecules are further functionalised with biomolecules in order to produce a selective biosensing surface.8,19 Stability of the anchor molecules during this further functionalisation process is vital for the development of graphene biosensing devices that require exposure to solvent or aqueous solut ...
... molecules are further functionalised with biomolecules in order to produce a selective biosensing surface.8,19 Stability of the anchor molecules during this further functionalisation process is vital for the development of graphene biosensing devices that require exposure to solvent or aqueous solut ...
1ST CHAPTER Long-questions-basic-concept
... is0.2 nm. Masses of atoms range from 10-27 to 10-25 kg. We can get an idea about the small size of an atom from the fact that a full stop may have two million atoms present in it. They are often expressed in atomic mass units (a.m.u). amu= 1.661x 10-24 g=1.661x10-27 kg Molecule: “The smallest partic ...
... is0.2 nm. Masses of atoms range from 10-27 to 10-25 kg. We can get an idea about the small size of an atom from the fact that a full stop may have two million atoms present in it. They are often expressed in atomic mass units (a.m.u). amu= 1.661x 10-24 g=1.661x10-27 kg Molecule: “The smallest partic ...
Full-Text PDF
... determination of H atom positions in protein crystal structures is restricted mainly due to their low X-ray scattering power, their inherent mobile nature, and limited crystallographic resolution [11]. 2. Neutron Crystallography and Hydrogen Atoms Neutron protein crystallography (NPX) is well-suited ...
... determination of H atom positions in protein crystal structures is restricted mainly due to their low X-ray scattering power, their inherent mobile nature, and limited crystallographic resolution [11]. 2. Neutron Crystallography and Hydrogen Atoms Neutron protein crystallography (NPX) is well-suited ...
Details of the scope analysis for each organism
... intermediate in the biosynthesis of Coenzyme A. N-((R)-4-Phosphopantothenoyl)-Lcysteine is formed from L-cysteine and D-4'-phosphopantothenate. The biosynthesis of L-cysteine requires Coenzyme A (Additional Fig. S4), consequently the biosynthesis of Coenzyme A is autocatalytic. The set of autocataly ...
... intermediate in the biosynthesis of Coenzyme A. N-((R)-4-Phosphopantothenoyl)-Lcysteine is formed from L-cysteine and D-4'-phosphopantothenate. The biosynthesis of L-cysteine requires Coenzyme A (Additional Fig. S4), consequently the biosynthesis of Coenzyme A is autocatalytic. The set of autocataly ...
No Slide Title
... Many of these mutations are positioned such that they could disrupt the secondary structure of the molecule. ...
... Many of these mutations are positioned such that they could disrupt the secondary structure of the molecule. ...
Atomic Mass: The atomic mass of an element is the mass average of
... Second: to determine the Empirical formula for ethanol: Steps ...
... Second: to determine the Empirical formula for ethanol: Steps ...
Exam 1
... Which one of the following statements about percentage of ethanol in the vapours shown at points X, Y and Z, when the temperature is at a constant 78°C, is true? A. The percentage of ethanol in the vapours at X is equal to 50%. B. The percentages of ethanol in the vapours increase in order at positi ...
... Which one of the following statements about percentage of ethanol in the vapours shown at points X, Y and Z, when the temperature is at a constant 78°C, is true? A. The percentage of ethanol in the vapours at X is equal to 50%. B. The percentages of ethanol in the vapours increase in order at positi ...
Empirical Formula, Molecular Formula, Percent Composition
... Compare both of your reactant amounts to the same product in this case Al2(SO4)3. Then find out how much products will be produced from each individual reactant. Whichever reactant yields the least amount of product that is your limiting reactant. 4 moles Al x 1 mole Al2(SO4)3 / 2 moles Al= 2 moles ...
... Compare both of your reactant amounts to the same product in this case Al2(SO4)3. Then find out how much products will be produced from each individual reactant. Whichever reactant yields the least amount of product that is your limiting reactant. 4 moles Al x 1 mole Al2(SO4)3 / 2 moles Al= 2 moles ...
Student Solutions Manual Errata
... liters. Notice that length and volume are different types of units. When the type of unit given and the unit in the answer are different (i.e., length and volume units) this often means you will need to use an equation. The key equation is the volume equation: ...
... liters. Notice that length and volume are different types of units. When the type of unit given and the unit in the answer are different (i.e., length and volume units) this often means you will need to use an equation. The key equation is the volume equation: ...
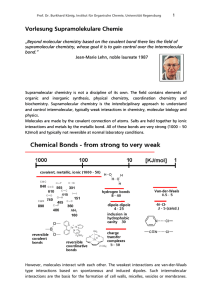
Vorlesung Supramolekulare Chemie
... directional and therefore of special importance in molecular recognition. The lengths, strengths and orientation of hydrogen bonds vary over a large range. In the solid state a single hydrogen bond can be sufficient to determine the structure; in solution or gas phase it may influence the aggregate ...
... directional and therefore of special importance in molecular recognition. The lengths, strengths and orientation of hydrogen bonds vary over a large range. In the solid state a single hydrogen bond can be sufficient to determine the structure; in solution or gas phase it may influence the aggregate ...
Significant Figures
... significant figures and are called exact numbers. An example would be a whole number from a balanced equation: 6.021 x 4/2 = 12.04 where 4/2 is perhaps the stoichiometric ratio of product to reactant. Since both 4 and 2 are exact numbers they can be considered to each have more significant figures t ...
... significant figures and are called exact numbers. An example would be a whole number from a balanced equation: 6.021 x 4/2 = 12.04 where 4/2 is perhaps the stoichiometric ratio of product to reactant. Since both 4 and 2 are exact numbers they can be considered to each have more significant figures t ...
Week 1 NEPHAR 201- Analytical Chemistry II_Introduction_5
... • Electroanalytical methods: methods that are based on quantifying the analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. ...
... • Electroanalytical methods: methods that are based on quantifying the analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. ...
Size-exclusion chromatography

Size-exclusion chromatography (SEC) is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.