Learner resource 1: Answers
... The chemical reactions within a cell by which ATP is produced from respiratory substrates such ...
... The chemical reactions within a cell by which ATP is produced from respiratory substrates such ...
CHAPTER 9
... 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Describe where pyruvate is oxidized ...
... 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Describe where pyruvate is oxidized ...
File
... 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Describe where pyruvate is oxidized ...
... 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Describe where pyruvate is oxidized ...
chapter 9
... 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Describe where pyruvate is oxidized ...
... 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Describe where pyruvate is oxidized ...
pentose phosphate pathway
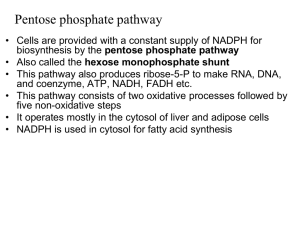
... Pentose phosphate pathway • Cells are provided with a constant supply of NADPH for biosynthesis by the pentose phosphate pathway • Also called the hexose monophosphate shunt • This pathway also produces ribose-5-P to make RNA, DNA, and coenzyme, ATP, NADH, FADH etc. • This pathway consists of two ox ...
... Pentose phosphate pathway • Cells are provided with a constant supply of NADPH for biosynthesis by the pentose phosphate pathway • Also called the hexose monophosphate shunt • This pathway also produces ribose-5-P to make RNA, DNA, and coenzyme, ATP, NADH, FADH etc. • This pathway consists of two ox ...
Answers - Shelton State
... 19. What is the anticodon for UUC? For what does this code? AAG; Phe 20. DNA is sometimes made from RNA by retro viruses. This process is called reverse transcription. 21. Nucleic acids are synthesized from their 5’ end to their 3’ end. 22. Define: antiparallel, termination code, enzyme, frame shift ...
... 19. What is the anticodon for UUC? For what does this code? AAG; Phe 20. DNA is sometimes made from RNA by retro viruses. This process is called reverse transcription. 21. Nucleic acids are synthesized from their 5’ end to their 3’ end. 22. Define: antiparallel, termination code, enzyme, frame shift ...
Glycolysis Reactions
... Glycolysis is the sequence of reactions that converts glucose into pyruvate with the concomitant production of a relatively small amount of ATP. Glycolysis can be carried out anerobically (in the absence of oxygen) and is thus an especially important pathway for organisms that can ferment sugars. Fo ...
... Glycolysis is the sequence of reactions that converts glucose into pyruvate with the concomitant production of a relatively small amount of ATP. Glycolysis can be carried out anerobically (in the absence of oxygen) and is thus an especially important pathway for organisms that can ferment sugars. Fo ...
BCOR 11 Exploring Biology
... B) the thermodynamically favorable flow of electrons from NADH to the mitochondrial electron transport carriers. C) the final transfer of electrons to oxygen. D) the difference in H+ concentrations on opposite sides of the inner mitochondrial membrane. E) the thermodynamically favorable transfer of ...
... B) the thermodynamically favorable flow of electrons from NADH to the mitochondrial electron transport carriers. C) the final transfer of electrons to oxygen. D) the difference in H+ concentrations on opposite sides of the inner mitochondrial membrane. E) the thermodynamically favorable transfer of ...
Outline05 Enzymes - Napa Valley College
... at high [S], reaction rate reaches a maximum level - at the saturation point, all active sites are occupied - maximum rate is limited by number of available enzymes [Substrate] 4. Regulation a. covalent regulation - regulation of enzyme via covalent binding of a chemical group - usually involves add ...
... at high [S], reaction rate reaches a maximum level - at the saturation point, all active sites are occupied - maximum rate is limited by number of available enzymes [Substrate] 4. Regulation a. covalent regulation - regulation of enzyme via covalent binding of a chemical group - usually involves add ...
Ch9CellularRespiration
... invertebrates in respirometer experiments has ethical implications (4.5). • Phosphorylation of molecules makes them less stable. Skill: Analysis of results from experiments involving measurement of • Energy released by oxidation reactions is carried to the cristae of respiration rates in germinating ...
... invertebrates in respirometer experiments has ethical implications (4.5). • Phosphorylation of molecules makes them less stable. Skill: Analysis of results from experiments involving measurement of • Energy released by oxidation reactions is carried to the cristae of respiration rates in germinating ...
File
... CoA is released to go back to the outer compartment. • This entire process consumes water (to get oxygen, making the process aerobic), and releases 6 NADH + H+ & 2 FADH2 molecules, 4 CO2 molecules per glucose molecule that we started with, and produces 2 ATP per glucose molecule that we started with ...
... CoA is released to go back to the outer compartment. • This entire process consumes water (to get oxygen, making the process aerobic), and releases 6 NADH + H+ & 2 FADH2 molecules, 4 CO2 molecules per glucose molecule that we started with, and produces 2 ATP per glucose molecule that we started with ...
Cellular Respiration
... Occurs in mitochondria; uses the high energy electrons captured in the Krebs Cycle (in NADH and FADH2) to form ATP and water. ...
... Occurs in mitochondria; uses the high energy electrons captured in the Krebs Cycle (in NADH and FADH2) to form ATP and water. ...
Principles of Metabolism
... the different energy couplers are interconvertible • For example, as we will see in oxidative energy metabolism, most of the potential energy of foodstuff is captured first as reduced coenzyme (NADH, FADH2), then converted to an electrochemical gradient of H+ across the mitochondrial inner membrane ...
... the different energy couplers are interconvertible • For example, as we will see in oxidative energy metabolism, most of the potential energy of foodstuff is captured first as reduced coenzyme (NADH, FADH2), then converted to an electrochemical gradient of H+ across the mitochondrial inner membrane ...
Alcoholic fermentation
... 13a) How does a cell use feedback inhibition to control its catabolism? As ATP concentration decreases, respiration speeds up; as ATP concentration increases, respiration slows down (regulated by enzymes) 13b) What is the role of the enzyme phosphofructokinase in this feedback inhibition? It catalyz ...
... 13a) How does a cell use feedback inhibition to control its catabolism? As ATP concentration decreases, respiration speeds up; as ATP concentration increases, respiration slows down (regulated by enzymes) 13b) What is the role of the enzyme phosphofructokinase in this feedback inhibition? It catalyz ...
cellular respiration
... and electron carriers • ATP and electron carriers are used up • Electron carriers power electron absorbing CO2 making transport chain which creates 3-carbon sugar in the proton gradient Calvin Cycle – Used by ATP Synthase • 3-carbon sugars made • Electrons are dumped onto O2 into glucose to make wat ...
... and electron carriers • ATP and electron carriers are used up • Electron carriers power electron absorbing CO2 making transport chain which creates 3-carbon sugar in the proton gradient Calvin Cycle – Used by ATP Synthase • 3-carbon sugars made • Electrons are dumped onto O2 into glucose to make wat ...
Photosynthesis
... the enzyme Rubisco. 3. One - 3 Carbon molecule known as G3P is formed for each Carbon dioxide(3) that gets fixed. It takes two turns of the cycle to produce ONE 6 carbon molecule of sugar. 4. ATP and NADPH are necessary to run this reaction and generate ADP, and NADP+ for the light reactions. ...
... the enzyme Rubisco. 3. One - 3 Carbon molecule known as G3P is formed for each Carbon dioxide(3) that gets fixed. It takes two turns of the cycle to produce ONE 6 carbon molecule of sugar. 4. ATP and NADPH are necessary to run this reaction and generate ADP, and NADP+ for the light reactions. ...
Microbial Metabolism
... joins with Oxaloacetic Acid (4C) to make Citric Acid (6C) Citric acid is oxidized releasing CO2 , free H+, & e- and forming ketoglutaric acid (5C) Free e- reduce the energy carriers NAD+ to NADH and FAD+ to FADH2 Ketoglutaric acid is also oxidized releasing more CO2 , free H+, & eThe cycle continues ...
... joins with Oxaloacetic Acid (4C) to make Citric Acid (6C) Citric acid is oxidized releasing CO2 , free H+, & e- and forming ketoglutaric acid (5C) Free e- reduce the energy carriers NAD+ to NADH and FAD+ to FADH2 Ketoglutaric acid is also oxidized releasing more CO2 , free H+, & eThe cycle continues ...
respiration - sandsbiochem
... 1. What is the chemical equation for cellular respiration? 2. Remember: OILRIG A. In the conversion of glucose and oxygen to CO2 and H2O, which molecule is reduced? B. Which is oxidized? C. What happens to the energy that is released in this redox reaction? 3. NAD+ is called a(n) ________________. ...
... 1. What is the chemical equation for cellular respiration? 2. Remember: OILRIG A. In the conversion of glucose and oxygen to CO2 and H2O, which molecule is reduced? B. Which is oxidized? C. What happens to the energy that is released in this redox reaction? 3. NAD+ is called a(n) ________________. ...
3.7 Cell Respiration
... Aerobic pathways use oxygen, use the link reaction, krebs cycle, electron transport chain, oxidative phosphorylation, and produces a large amount of ATP (36). Anaerobic pathways do not require oxygen, produce lactic acid/lactate through lactic acid fermentation, produces ethanol through alcoholic fe ...
... Aerobic pathways use oxygen, use the link reaction, krebs cycle, electron transport chain, oxidative phosphorylation, and produces a large amount of ATP (36). Anaerobic pathways do not require oxygen, produce lactic acid/lactate through lactic acid fermentation, produces ethanol through alcoholic fe ...
Preview from Notesale.co.uk Page 3 of 61
... The process of breaking a glucose molecule into two pyruvic acid molecules. The process of breaking down pyruvic acid into carbon dioxide. High energy electrons are used to convert ADP to ATP. The organelle in which cellular respiration takes place. The release of energy from food without the presen ...
... The process of breaking a glucose molecule into two pyruvic acid molecules. The process of breaking down pyruvic acid into carbon dioxide. High energy electrons are used to convert ADP to ATP. The organelle in which cellular respiration takes place. The release of energy from food without the presen ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.