1st Semester Final Exam Study Guide (excluding DNA/protein
... 12. Which of these often serve as receptors or cell recognition molecules on cell surfaces? a) transmembrane proteins b) integral proteins c) peripheral proteins d) integrins e) glycoproteins 13. Approximately how many molecules of ATP are produced from the complete oxidation of two molecules of gl ...
... 12. Which of these often serve as receptors or cell recognition molecules on cell surfaces? a) transmembrane proteins b) integral proteins c) peripheral proteins d) integrins e) glycoproteins 13. Approximately how many molecules of ATP are produced from the complete oxidation of two molecules of gl ...
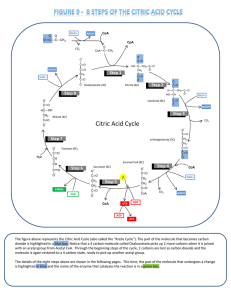
Citric Acid Cycle - BYU
... The CH3 end of the acetyl CoA loses a proton and becomes bonded to the second carbonyl carbon (C=O) of oxyloacetate. The coenzyme (CoA) is subsequently lost with the input of ...
... The CH3 end of the acetyl CoA loses a proton and becomes bonded to the second carbonyl carbon (C=O) of oxyloacetate. The coenzyme (CoA) is subsequently lost with the input of ...
Lecture Fermentation
... Rumen epithelium not protected by mucous Acid causes inflammation and ulceration (rumenitis) Lactate promotes growth of Fusobacterium necrophorum Fus. necrophorum infects ruminal ulcers If Fus. necrophorum pass from rumen to blood, they colonize in the liver causing abscesses Incidence of liver absc ...
... Rumen epithelium not protected by mucous Acid causes inflammation and ulceration (rumenitis) Lactate promotes growth of Fusobacterium necrophorum Fus. necrophorum infects ruminal ulcers If Fus. necrophorum pass from rumen to blood, they colonize in the liver causing abscesses Incidence of liver absc ...
The pool of ADP and ATP regulates anaerobic
... Since no NAD⫹ is regenerated in the acetate formation pathway, one ethanol molecule must be formed for every acetate molecule to regenerate two NAD⫹ molecules and maintain the redox balance. It is important to note that LDH under anaerobic conditions competes with two enzymes for its substrates; it ...
... Since no NAD⫹ is regenerated in the acetate formation pathway, one ethanol molecule must be formed for every acetate molecule to regenerate two NAD⫹ molecules and maintain the redox balance. It is important to note that LDH under anaerobic conditions competes with two enzymes for its substrates; it ...
cellular respiration webquest
... 2. What living things carry on the process of cellular respiration? (search through google or bing) ____________________________________________________________________________ ____________________________________________________________________________ 3. Write the chemical equation for cellular re ...
... 2. What living things carry on the process of cellular respiration? (search through google or bing) ____________________________________________________________________________ ____________________________________________________________________________ 3. Write the chemical equation for cellular re ...
Nucleotide Metabolism
... OMP Decarboxylase is one of the Most Efficient Enzymes Known and Makes UMP CTP is Synthesized from UTP by CTP Synthetase CTP Synthetase Activated by GTP, Inhibited by CTP and Phosphorylation CTP Synthetase Helps to Balance Purines and Pyrimidines ...
... OMP Decarboxylase is one of the Most Efficient Enzymes Known and Makes UMP CTP is Synthesized from UTP by CTP Synthetase CTP Synthetase Activated by GTP, Inhibited by CTP and Phosphorylation CTP Synthetase Helps to Balance Purines and Pyrimidines ...
Effect of Growth Rate and Nutrient Limitation on the
... The adenylate pool is maintained by the action of various enzymes. Adenylate kinase brings the three nucleotides into equilibrium (2ADP * AMP + ATP) an! while its specific activity was largely unaffected by dilution rate under nitrogen and oxygen limitation, it was inversely related to dilution rate ...
... The adenylate pool is maintained by the action of various enzymes. Adenylate kinase brings the three nucleotides into equilibrium (2ADP * AMP + ATP) an! while its specific activity was largely unaffected by dilution rate under nitrogen and oxygen limitation, it was inversely related to dilution rate ...
Chapter 8
... transforms matter and energy, subject to the laws of thermodynamics • Metabolism is the totality of an organism’s chemical reactions • Metabolism is an emergent property of life that arises from interactions between molecules within the cell ...
... transforms matter and energy, subject to the laws of thermodynamics • Metabolism is the totality of an organism’s chemical reactions • Metabolism is an emergent property of life that arises from interactions between molecules within the cell ...
Protein Metabolism - Morning By Morning!
... Produce glutamate – may be deaminated to yield ammonia for urea cycle. Can be converted to glucose (alanine-glucose cycle) – transport N to liver for conversion to urea while also generating needed substrate. Occurs in low CHO stores (liver glycogen) to maintain blood glucose; eExcessive use for glu ...
... Produce glutamate – may be deaminated to yield ammonia for urea cycle. Can be converted to glucose (alanine-glucose cycle) – transport N to liver for conversion to urea while also generating needed substrate. Occurs in low CHO stores (liver glycogen) to maintain blood glucose; eExcessive use for glu ...
The b-oxidation pathway as an energy source
... Cytochrome c has a dual function in the cell. Electron transport for ATP production AND the major cause of most programmed cell death (apoptosis) is initiated by the release of cytochrome c into the cytosol! ...
... Cytochrome c has a dual function in the cell. Electron transport for ATP production AND the major cause of most programmed cell death (apoptosis) is initiated by the release of cytochrome c into the cytosol! ...
Student notes in ppt
... acids when glucose levels are high and the amount of acetyl CoA produced exceeds the energy requirements of the cell. Glucose provides the necessary substrates for triacylglycerol synthesis (acetyl CoA for fatty acid synthesis and glycerol) using reactions in the glycolytic pathway and the citrate c ...
... acids when glucose levels are high and the amount of acetyl CoA produced exceeds the energy requirements of the cell. Glucose provides the necessary substrates for triacylglycerol synthesis (acetyl CoA for fatty acid synthesis and glycerol) using reactions in the glycolytic pathway and the citrate c ...
Gluconeogenesis
... Because oxaloacetate cannot be transported across the mitochondrial membrane, it must be reduced to malate, transported to the cytosol, and then oxidized back to oxaloacetate before gluconeogenesis can continue. ...
... Because oxaloacetate cannot be transported across the mitochondrial membrane, it must be reduced to malate, transported to the cytosol, and then oxidized back to oxaloacetate before gluconeogenesis can continue. ...
Anaerobic Respiration
... Fermentation occurs in the cytoplasm of the cell, and produces NAD+ and ATP. ...
... Fermentation occurs in the cytoplasm of the cell, and produces NAD+ and ATP. ...
Nucleotide Metabolism - Oregon State University
... OMP Decarboxylase is one of the Most Efficient Enzymes Known and Makes UMP CTP is Synthesized from UTP by CTP Synthetase CTP Synthetase Activated by GTP, Inhibited by CTP and Phosphorylation CTP Synthetase Helps to Balance Purines and Pyrimidines ...
... OMP Decarboxylase is one of the Most Efficient Enzymes Known and Makes UMP CTP is Synthesized from UTP by CTP Synthetase CTP Synthetase Activated by GTP, Inhibited by CTP and Phosphorylation CTP Synthetase Helps to Balance Purines and Pyrimidines ...
presentation source
... Sulphonyl ureas bind to the SUR1 protein associated with the K+ channel and like ATP induces closure. Glucose ...
... Sulphonyl ureas bind to the SUR1 protein associated with the K+ channel and like ATP induces closure. Glucose ...
Chapter 7 7 The Behavior of Proteins: Enzymes Mechanisms and
... id ring i iis where h reduction-oxidation d i id i occurs ...
... id ring i iis where h reduction-oxidation d i id i occurs ...
File
... resources of the cell. Some metabolic pathways release energy by breaking down complex molecules to simpler compounds. These degradative processes are called catabolic pathways, or breakdown pathways. A major pathway of catabolism is cellular respiration, in which the sugar glucose and other organic ...
... resources of the cell. Some metabolic pathways release energy by breaking down complex molecules to simpler compounds. These degradative processes are called catabolic pathways, or breakdown pathways. A major pathway of catabolism is cellular respiration, in which the sugar glucose and other organic ...
Fatty Acid Biosynthesis
... • AcetylCoA generated from pyruvate by the action of PDH and by β-oxidation of fatty acids is in the mitochondria. • For fatty acid biosynthesis, acetylCoA has to be transported from the mitochondria to the cytoplasm. This is done via a shuttle system called the Citrate Shuttle. • AcetylCoA reacts w ...
... • AcetylCoA generated from pyruvate by the action of PDH and by β-oxidation of fatty acids is in the mitochondria. • For fatty acid biosynthesis, acetylCoA has to be transported from the mitochondria to the cytoplasm. This is done via a shuttle system called the Citrate Shuttle. • AcetylCoA reacts w ...
4. Power: Pathways that make ATP
... gasoline is used to move the pistons, which then causes the wheels to move. This process uses O2 and an equation of the reaction of gasoline with oxygen, O2, is the same as what occurs in metabolism: C,H (gasoline in cars, food in people) + O2 CO2 + H2O + energy The above reaction is said to be ae ...
... gasoline is used to move the pistons, which then causes the wheels to move. This process uses O2 and an equation of the reaction of gasoline with oxygen, O2, is the same as what occurs in metabolism: C,H (gasoline in cars, food in people) + O2 CO2 + H2O + energy The above reaction is said to be ae ...
Metabolic Acidosis
... • The ratio of phosphorus to oxygen is 3:1 • 6 ATP can be produced per O2 • Consumption of at rest is close to 12 mmol/min • The amount of ATP needed per minute is 12 X 6, or 72 mmol/min ...
... • The ratio of phosphorus to oxygen is 3:1 • 6 ATP can be produced per O2 • Consumption of at rest is close to 12 mmol/min • The amount of ATP needed per minute is 12 X 6, or 72 mmol/min ...
... electrophoretic technique permitted the study of enzyme activity in relatively small amounts of crude cell-free extract. O’Brien et al. (1981, 1983) have reported the presence of PPase in each of 15 Mycoplasma species tested, and in three of five Acholeplasma species. They did not find the enzyme ac ...
AMINO ACIDS METABOLISM ** Dr. Mohammed Abdullateef **
... blood/brain barrier → increased synthesis of glutamate from a-ketoglutarate by glutamate dehydrogenase, increased synthesis of glutamine. Alpha ketoglutarate is consumed and not available to reach other processes such as TCA cycle. This causes decreased ATP production. Brain is more affected as it d ...
... blood/brain barrier → increased synthesis of glutamate from a-ketoglutarate by glutamate dehydrogenase, increased synthesis of glutamine. Alpha ketoglutarate is consumed and not available to reach other processes such as TCA cycle. This causes decreased ATP production. Brain is more affected as it d ...
Lactic Acid in Muscle and its Effects on meat Quality(3)
... In muscle, the main sources of glucose are blood glucose and glycogen stored in muscles. In stress, hormones and/or physical stress activate phosphorylase that is able to cleave glucosyl units from a glycogen molecule at an enormous speed. The reaction cascade is explicitly described in every textbo ...
... In muscle, the main sources of glucose are blood glucose and glycogen stored in muscles. In stress, hormones and/or physical stress activate phosphorylase that is able to cleave glucosyl units from a glycogen molecule at an enormous speed. The reaction cascade is explicitly described in every textbo ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.