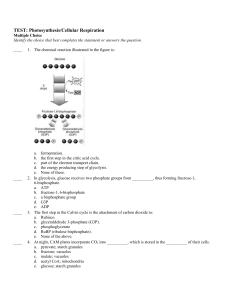
form/activity - Science of Security
... • “..and to build engines.” (propulsion) • “Inability to balance/steer [is the] problem.” (control) • “When this one feature has been worked out, the age of flying will have arrived, for all other difficulties are of minor importance.” ...
... • “..and to build engines.” (propulsion) • “Inability to balance/steer [is the] problem.” (control) • “When this one feature has been worked out, the age of flying will have arrived, for all other difficulties are of minor importance.” ...
Principles of Metabolic Regulation
... require energy for extended periods of time. For example, ducks generally fly several thousand miles during their annual migration. The flight muscles of migratory birds have a high oxidative capacity and obtain the necessary ATP through the oxidation of acetyl-CoA (obtained from fats) via the citri ...
... require energy for extended periods of time. For example, ducks generally fly several thousand miles during their annual migration. The flight muscles of migratory birds have a high oxidative capacity and obtain the necessary ATP through the oxidation of acetyl-CoA (obtained from fats) via the citri ...
Comparison of the Functional Differences for the Homologous Residues within... Carboxy Phosphate and Carbamate Domains of Carbamoyl Phosphate Synthetase
... carbamoyl phosphate from two molecules of MgATP, bicarbonate, and glutamine. It has been previously shown that the amino- and carboxy-terminal halves of the large subunit of this protein are homologous. A working model for the active site structure of the carboxy-terminal domain of the large subunit ...
... carbamoyl phosphate from two molecules of MgATP, bicarbonate, and glutamine. It has been previously shown that the amino- and carboxy-terminal halves of the large subunit of this protein are homologous. A working model for the active site structure of the carboxy-terminal domain of the large subunit ...
biochemical model for enhanced biological phosphorus removal
... in the next section. Three major mechanisms are used by most bacteria to translocate protons and maintain a pmf (see Fig. 4). The first one is of major importance and makes use of the cytoplasmic membrane-bound electron transport chain to expel H + from the cell when carbon substrates and an electro ...
... in the next section. Three major mechanisms are used by most bacteria to translocate protons and maintain a pmf (see Fig. 4). The first one is of major importance and makes use of the cytoplasmic membrane-bound electron transport chain to expel H + from the cell when carbon substrates and an electro ...
Fatty Acid Oxid
... Carnitine-mediated transfer of the fatty acyl moiety into the mitochondrial matrix is a 3-step process: 1. Carnitine Palmitoyl Transferase I, an enzyme on the cytosolic surface of the outer mitochondrial membrane, transfers a fatty acid from CoA to the OH on carnitine. 2. An antiporter in the inner ...
... Carnitine-mediated transfer of the fatty acyl moiety into the mitochondrial matrix is a 3-step process: 1. Carnitine Palmitoyl Transferase I, an enzyme on the cytosolic surface of the outer mitochondrial membrane, transfers a fatty acid from CoA to the OH on carnitine. 2. An antiporter in the inner ...
11. PHOTOSYNTHETIC PATHWAYS - Development of e
... diphosphate is regenerated. In Calvin cycle, 12 NADPH2 and 18 ATPs are required to fix 6 CO2 molecules into one hexose sugar molecule (fructose 6 phosphate). 6 CO2 + 12 NADPH2 + 18 ATP ...
... diphosphate is regenerated. In Calvin cycle, 12 NADPH2 and 18 ATPs are required to fix 6 CO2 molecules into one hexose sugar molecule (fructose 6 phosphate). 6 CO2 + 12 NADPH2 + 18 ATP ...
WRL3116.tmp
... C. * Interactions between polar functional groups on the substrate surface and hydrophobic amino acids in the enzyme’s substrate binding site. D. A and b E. A and c 71. Which of the following is characteristic of an enzyme catalyst? A. It positions reactants in the correct orientation B. It lowers t ...
... C. * Interactions between polar functional groups on the substrate surface and hydrophobic amino acids in the enzyme’s substrate binding site. D. A and b E. A and c 71. Which of the following is characteristic of an enzyme catalyst? A. It positions reactants in the correct orientation B. It lowers t ...
A SOLUBLE RIBONUCLEIC ACID INTERMEDIATE IN PROTEIN
... irreversibly into cY-peptide linkage in protein has been used in our laboratories for a number of years as a measure of protein synthesis. The essential components of this system are the microsomal ribonucleoprotein particles, certain enzymes derived from the soluble protein fraction, adenosine trip ...
... irreversibly into cY-peptide linkage in protein has been used in our laboratories for a number of years as a measure of protein synthesis. The essential components of this system are the microsomal ribonucleoprotein particles, certain enzymes derived from the soluble protein fraction, adenosine trip ...
Events of The Krebs Cycle
... Cellular Respiration Cellular respiration is a group of catabolic reactions in the cell that breaks down food fuels such as glucose. These reactions can be grouped into metabolic processes called glycolysis, the Krebs cycle and the electron transport system. The main purpose of cellular respiration ...
... Cellular Respiration Cellular respiration is a group of catabolic reactions in the cell that breaks down food fuels such as glucose. These reactions can be grouped into metabolic processes called glycolysis, the Krebs cycle and the electron transport system. The main purpose of cellular respiration ...
enzymes - Yengage
... Function in dilute aqueous solutions under mild conditions of temperature and pH Physiological regulation Several enzymes can work together in a specific order creating - metabolic pathways ...
... Function in dilute aqueous solutions under mild conditions of temperature and pH Physiological regulation Several enzymes can work together in a specific order creating - metabolic pathways ...
41 Purine and Pyrimidine Metabolism
... because most of the ingested nucleic acids are metabolized by the intestinal epithelial cells. The de novo pathway of purine synthesis is complex, consisting of 11 steps, and requiring 6 molecules of ATP for every purine synthesized. The precursors that donate components to produce purine nucleotide ...
... because most of the ingested nucleic acids are metabolized by the intestinal epithelial cells. The de novo pathway of purine synthesis is complex, consisting of 11 steps, and requiring 6 molecules of ATP for every purine synthesized. The precursors that donate components to produce purine nucleotide ...
TCA cycle cross products (also known as “nothing is simple” My
... This is multistep reaction carried out by a large complex; Reactome breaks this down into several steps. GO:0050243 has NADP+ I have at least 4 textbooks in front of me indicating that this reaction uses NAD, not NADP. But, I see a lot of papers indicating NADP; So, I suggest the def be altered to p ...
... This is multistep reaction carried out by a large complex; Reactome breaks this down into several steps. GO:0050243 has NADP+ I have at least 4 textbooks in front of me indicating that this reaction uses NAD, not NADP. But, I see a lot of papers indicating NADP; So, I suggest the def be altered to p ...
Chapter 17. Amino Acid Oxidation and the Production of Urea
... (transamination and oxidative decarboxylation) of all these three amino acids in extrahepatic tissues. • The a-keto acid dehydrogenase complex has a similar structure and catalyzes essentially the same type of reaction (oxidative decarboxylation of aketo acids) as pyruvate dehydrogenase complex and ...
... (transamination and oxidative decarboxylation) of all these three amino acids in extrahepatic tissues. • The a-keto acid dehydrogenase complex has a similar structure and catalyzes essentially the same type of reaction (oxidative decarboxylation of aketo acids) as pyruvate dehydrogenase complex and ...
Structure of Mammalian AMPK and its regulation by ADP
... bind NADH, that is responsible for protection against dephosphorylation. They also carried out co-crystallization of the regulatory fragment with one molar equivalent of ADP The resulting electron density map showed full occupancy of ADP at site 1 and no detectable density at site 3, identifying ...
... bind NADH, that is responsible for protection against dephosphorylation. They also carried out co-crystallization of the regulatory fragment with one molar equivalent of ADP The resulting electron density map showed full occupancy of ADP at site 1 and no detectable density at site 3, identifying ...
Metabolism
... cofactors Compounds required for an enzyme to be active. Cofactors include coenzymes and metal ions such as iron (Fe2+), copper (Cu2+), and magnesium (Mg2+). coenzymes Organic compounds, often B vitamin derivatives, that combine with an inactive enzyme to form an active enzyme. Coenzymes associate ...
... cofactors Compounds required for an enzyme to be active. Cofactors include coenzymes and metal ions such as iron (Fe2+), copper (Cu2+), and magnesium (Mg2+). coenzymes Organic compounds, often B vitamin derivatives, that combine with an inactive enzyme to form an active enzyme. Coenzymes associate ...
ETs08
... of mitochondrial inner membrane into its interior That passage actually generates both chemical and electrical energy. This is because they are moving down a concentration and electricalpotential gradient. ...
... of mitochondrial inner membrane into its interior That passage actually generates both chemical and electrical energy. This is because they are moving down a concentration and electricalpotential gradient. ...
Use to make Test Corrections (Answer in complete sentence +10 pts
... Which process does not match the starting materials? a. formation of acetyl CoAcitric acid, CO2, NADH b. citric acid cycleacetyl CoA, H2O, NAD+, FAD, ADP, Pi c. electron transport and chemiosmosisNADH, FADH2, O2, ADP, Pi d. glycolysisglucose, ATP, NAD+, ADP, Pi e. All of these processes match th ...
... Which process does not match the starting materials? a. formation of acetyl CoAcitric acid, CO2, NADH b. citric acid cycleacetyl CoA, H2O, NAD+, FAD, ADP, Pi c. electron transport and chemiosmosisNADH, FADH2, O2, ADP, Pi d. glycolysisglucose, ATP, NAD+, ADP, Pi e. All of these processes match th ...
Unit 4 Notes
... a net gain of ATP and reduced NAD • Pyruvate combines with coenzyme A in the link reaction to produce acetylcoenzyme A • Acetylcoenzyme A is effectively a two carbon molecule that combines with a four carbon molecule to produce a six carbon molecule which enters the Krebs cycle. In a series of oxida ...
... a net gain of ATP and reduced NAD • Pyruvate combines with coenzyme A in the link reaction to produce acetylcoenzyme A • Acetylcoenzyme A is effectively a two carbon molecule that combines with a four carbon molecule to produce a six carbon molecule which enters the Krebs cycle. In a series of oxida ...
5-1 Necleotide Metabolism (purine)
... (the reutilization of bases from dietary or catabolic sources) ...
... (the reutilization of bases from dietary or catabolic sources) ...
AP UNIT 3
... molecules and O2 and yields ATP • Anaerobic respiration is similar to aerobic respiration but consumes compounds other than O2 Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings ...
... molecules and O2 and yields ATP • Anaerobic respiration is similar to aerobic respiration but consumes compounds other than O2 Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings ...
Mechanistic model of cardiac energy metabolism predicts
... domain consisting of blood plasma and interstitial fluid, which for simplicity is called “blood.” Within the extravascular tissue cells, we distinguished two domains: the cytosol and mitochondria. Between domains (blood-cytosol and cytosol-mitochondria), molecular transport can occur by passive diff ...
... domain consisting of blood plasma and interstitial fluid, which for simplicity is called “blood.” Within the extravascular tissue cells, we distinguished two domains: the cytosol and mitochondria. Between domains (blood-cytosol and cytosol-mitochondria), molecular transport can occur by passive diff ...
Cell Energy (GPC)
... The challenge for all living organisms is to obtain energy from their surroundings in forms that they can transfer or transform into usable energy to do work. Living cells have evolved to meet this challenge. Chemical energy stored within organic molecules such as sugars and fats is transferred and ...
... The challenge for all living organisms is to obtain energy from their surroundings in forms that they can transfer or transform into usable energy to do work. Living cells have evolved to meet this challenge. Chemical energy stored within organic molecules such as sugars and fats is transferred and ...
... iii) The energetic cost to insert the polar mainchain atoms into the bilayer is unfavorable, (by +1 kcal/mol). Therefore the sidechain must be large enough such that the transfer energy exceeds this. Anything larger than Ala will do. 9. (6 pts) Please do one of the following choices. Choice A: Brief ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.