Chapter 4: Aqueous Reactions and Solution
... B. Use the molecular equation to obtain the net-ionic equation: 1. Identify all the STRONG ELECTROLYTES. Search only the (aq) species for the strong electrolytes! 2. Rewrite the strong electrolytes as separated ions. All other species are rewritten without modification. 3. Cancel any ions that app ...
... B. Use the molecular equation to obtain the net-ionic equation: 1. Identify all the STRONG ELECTROLYTES. Search only the (aq) species for the strong electrolytes! 2. Rewrite the strong electrolytes as separated ions. All other species are rewritten without modification. 3. Cancel any ions that app ...
Practical Exercises in Physical Chemistry
... Mn(III) tris-oxalate. Adjust the photometer with distilled water for this band at 18 0C. Prepare the aqueous solution of Mn(III) tris-oxalate as described above and measure the time dependent extinction stepwise with the step size equal to 60 s as long as E ≥ 0.1 . In this experiment you do not need ...
... Mn(III) tris-oxalate. Adjust the photometer with distilled water for this band at 18 0C. Prepare the aqueous solution of Mn(III) tris-oxalate as described above and measure the time dependent extinction stepwise with the step size equal to 60 s as long as E ≥ 0.1 . In this experiment you do not need ...
Chemistry Unit 3 Holiday Homework Questions
... 10. When sodium is placed on water it reacts violently to produce hydrogen gas and a solution of sodium hydroxide. (a) Write a balanced chemical equation for this reaction. (b) If 9.2 g of sodium reacts completely, calculate the mass of gas that would be produced. Ans: 0.4g (c) What mass of water wo ...
... 10. When sodium is placed on water it reacts violently to produce hydrogen gas and a solution of sodium hydroxide. (a) Write a balanced chemical equation for this reaction. (b) If 9.2 g of sodium reacts completely, calculate the mass of gas that would be produced. Ans: 0.4g (c) What mass of water wo ...
Reaction Stoichiometry
... propellant. The equation is: N2H4(l) + 2H2O2(l) → N2(g) + 4H2O(g) Which is the limiting reactant in this reaction when 0.750 mol hydrazine is mixed with 0.500 mol hydrogen peroxide? ...
... propellant. The equation is: N2H4(l) + 2H2O2(l) → N2(g) + 4H2O(g) Which is the limiting reactant in this reaction when 0.750 mol hydrazine is mixed with 0.500 mol hydrogen peroxide? ...
Introduction
... the reaction. The stoichiometry is an essential component of the equation if we are to perform calculations such as determining the mass of water formed from a mixture of hydrogen and oxygen. Remember that atoms are not created or destroyed in reactions, but they are traded. This means that the numb ...
... the reaction. The stoichiometry is an essential component of the equation if we are to perform calculations such as determining the mass of water formed from a mixture of hydrogen and oxygen. Remember that atoms are not created or destroyed in reactions, but they are traded. This means that the numb ...
2nd Nine Weeks Notes
... 1. The first step in understanding how a given chemical reaction occurs is to determine the form of the rate law. 2. We must determine experimentally the power to which each reactant concentration must be raised in the rate law. a. An exponent of “1” is referred to as first order. b. An exponent of ...
... 1. The first step in understanding how a given chemical reaction occurs is to determine the form of the rate law. 2. We must determine experimentally the power to which each reactant concentration must be raised in the rate law. a. An exponent of “1” is referred to as first order. b. An exponent of ...
Revision IB2 Topic 1
... The reaction of ethanal and oxygen can be represented by the unbalanced equation below. __ CH3CHO + __ O2 → __ CO2 + __ H2O When the equation is balanced using the smallest possible integers, what is the coefficient for O2? ...
... The reaction of ethanal and oxygen can be represented by the unbalanced equation below. __ CH3CHO + __ O2 → __ CO2 + __ H2O When the equation is balanced using the smallest possible integers, what is the coefficient for O2? ...
program
... make a connection between bond types, lattice type, and the properties of a substance: • melting point and boiling point; • hardness and brittleness; • absence or presence of electrical conductivity in the solid, liquid, and/or dissolved ...
... make a connection between bond types, lattice type, and the properties of a substance: • melting point and boiling point; • hardness and brittleness; • absence or presence of electrical conductivity in the solid, liquid, and/or dissolved ...
Section 2 Chemical Formulas and Equations
... atom in the reactants becomes part of the products. When writing a chemical equation, make sure that the total number of atoms of each element in the reactants equals the total number of atoms of that element in the products. This process is called balancing the equation. Balancing equations comes f ...
... atom in the reactants becomes part of the products. When writing a chemical equation, make sure that the total number of atoms of each element in the reactants equals the total number of atoms of that element in the products. This process is called balancing the equation. Balancing equations comes f ...
Language of chemistry
... chemistry students to get themselves familiar with the language used by chemists. This will help the student while studying the subject. You will be introduced in this unit to the language used in chemistry. Let us take a look at some of the common terms often encountered in chemistry. Atom – An ato ...
... chemistry students to get themselves familiar with the language used by chemists. This will help the student while studying the subject. You will be introduced in this unit to the language used in chemistry. Let us take a look at some of the common terms often encountered in chemistry. Atom – An ato ...
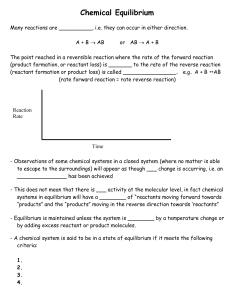
Chemical Equilibrium Equilibrium A state where the reactants and
... Knowing the equilibrium constant allows us to predict several important features of the reaction. 1) the tendency of the reaction to ___________ (but not the _______________) 2) whether a given set of concentrations represent an __________________ condition 3) the equilibrium position that will be ...
... Knowing the equilibrium constant allows us to predict several important features of the reaction. 1) the tendency of the reaction to ___________ (but not the _______________) 2) whether a given set of concentrations represent an __________________ condition 3) the equilibrium position that will be ...
Final Exam - Dawson College
... 3. In order to obtain full credit, you must show the method used to solve all problems involving calculations and express your answers to the correct number of significant figures. ...
... 3. In order to obtain full credit, you must show the method used to solve all problems involving calculations and express your answers to the correct number of significant figures. ...
Figure 1.01a: (a.)The surface of a single grain of table salt.
... mass of each element per mole of compound. Determine the # of moles of each element /mol compound. The integers of # of moles of each element are the subscript in the molecular formula. ...
... mass of each element per mole of compound. Determine the # of moles of each element /mol compound. The integers of # of moles of each element are the subscript in the molecular formula. ...
Chapter 1-3 Exam Review
... 2. I can determine the number of protons, neutrons and electrons in isotopes and in ions. 3. describe the works of John Dalton, J.J. Thomson (cathode ray tube), Robert Millikan (Oil Drop Experiment) and Ernst Rutherford (Gold Foil Experiment). 4. use the periodic table to predict the charges of mona ...
... 2. I can determine the number of protons, neutrons and electrons in isotopes and in ions. 3. describe the works of John Dalton, J.J. Thomson (cathode ray tube), Robert Millikan (Oil Drop Experiment) and Ernst Rutherford (Gold Foil Experiment). 4. use the periodic table to predict the charges of mona ...