Chemistry Notes for the Whole Year Powerpoint
... of the second element, after the first element, take off its last syllable and add -ide. • You also must use prefixes with covalent compounds. These prefixes denote how many atoms of each element are in the compounds. The prefixes are: mono (1, use on second element only), di (2), tri(3), tetra (4), ...
... of the second element, after the first element, take off its last syllable and add -ide. • You also must use prefixes with covalent compounds. These prefixes denote how many atoms of each element are in the compounds. The prefixes are: mono (1, use on second element only), di (2), tri(3), tetra (4), ...
Solutions
... ‣ If the number goes up, the species has been oxidized. ‣ If the number goes down it’s been reduced. ‣ Oxidation numbers can be positive or negative. ‣ Finding oxidation Numbers: ...
... ‣ If the number goes up, the species has been oxidized. ‣ If the number goes down it’s been reduced. ‣ Oxidation numbers can be positive or negative. ‣ Finding oxidation Numbers: ...
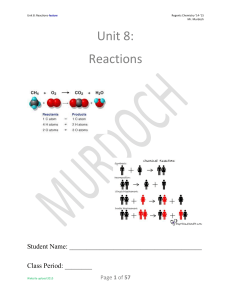
Unit 8: Reactions
... If you are given the names in your equation, the steps for writing a chemical equation are as follows: 1. Balance the Equation: i. Write in PENCIL (you’ll make mistakes; we all do!); ii. Write the coefficients in one element at a time; iii. You may only add coefficients; you may NOT change any chemi ...
... If you are given the names in your equation, the steps for writing a chemical equation are as follows: 1. Balance the Equation: i. Write in PENCIL (you’ll make mistakes; we all do!); ii. Write the coefficients in one element at a time; iii. You may only add coefficients; you may NOT change any chemi ...
chemical change
... Acetic acid is known to contain the elements carbon, hydrogen and oxygen only. A 4.24 g sample of acetic acid is combusted to form 6.21 g of carbon dioxide, and 2.54 g of water. Calculate the percentage composition of acetic acid, and use this to determine the empirical formula. ...
... Acetic acid is known to contain the elements carbon, hydrogen and oxygen only. A 4.24 g sample of acetic acid is combusted to form 6.21 g of carbon dioxide, and 2.54 g of water. Calculate the percentage composition of acetic acid, and use this to determine the empirical formula. ...
Section 4.8: Acid-Base Reactions
... Two compounds react to form two new compounds. All double replacement reactions must have a "driving force" that removes a pair of ions from solution. Ions in a precipitation reaction will keep their same charges as reactants and products. Formation of a precipitate: A precipitate is an insoluble su ...
... Two compounds react to form two new compounds. All double replacement reactions must have a "driving force" that removes a pair of ions from solution. Ions in a precipitation reaction will keep their same charges as reactants and products. Formation of a precipitate: A precipitate is an insoluble su ...
File
... • C12H22O11 (s) C12H22O11 (aq) • NO dissociation because NO ions • Sucrose dissolves in water because sugar is polar (-OH group), but dissociation does not occur. Sucrose molecules are simply separated from each other. No ions are formed ...
... • C12H22O11 (s) C12H22O11 (aq) • NO dissociation because NO ions • Sucrose dissolves in water because sugar is polar (-OH group), but dissociation does not occur. Sucrose molecules are simply separated from each other. No ions are formed ...
as a PDF
... trace metal Me studied in the respective aqueous system. If the data are not known they can be determined equally as well by voltammetry, as has been ...
... trace metal Me studied in the respective aqueous system. If the data are not known they can be determined equally as well by voltammetry, as has been ...
PRACTICE – Naming and Writing Ionic Compounds
... 2. The incandescent white of a fireworks display is caused by the reaction of phosphorus with oxygen to give tetraphosphorus decoxide. a. Write the balanced chemical equation for the reaction. ...
... 2. The incandescent white of a fireworks display is caused by the reaction of phosphorus with oxygen to give tetraphosphorus decoxide. a. Write the balanced chemical equation for the reaction. ...
SAMPLE QUESTION PAPER SIR.S.M.TAHIR CHEMISTRY Mob: 9557076999
... In an increasing order of basic strength. C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2 ...
... In an increasing order of basic strength. C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2 ...
IV Stoichiometry - s3.amazonaws.com
... Na2SO4 • XH2O(s) was heated to remove the water. After heating, 7.95 grams of material remained. What is the formula of the hydrate: (find the value of X) A) Na2SO4 • H2O B) Na2SO4 • 2H2O C) Na2SO4 • 3H2O D) Na2SO4 • 5H2O E) Na2SO4 • 7H2O ...
... Na2SO4 • XH2O(s) was heated to remove the water. After heating, 7.95 grams of material remained. What is the formula of the hydrate: (find the value of X) A) Na2SO4 • H2O B) Na2SO4 • 2H2O C) Na2SO4 • 3H2O D) Na2SO4 • 5H2O E) Na2SO4 • 7H2O ...
Test bank questions
... At 700 K, the reaction 2SO2(g) + O2(g) 2SO3(g) has the equilibrium constant Kc = 4.3 106, and the following concentrations are present: [SO2] = 0.010 M; [SO3] = 10. M; [O2] = 0.010 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to rig ...
... At 700 K, the reaction 2SO2(g) + O2(g) 2SO3(g) has the equilibrium constant Kc = 4.3 106, and the following concentrations are present: [SO2] = 0.010 M; [SO3] = 10. M; [O2] = 0.010 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to rig ...
AP 2005 Chemistry Free-Response Questions
... (a) Write the equilibrium-constant expression for the reaction. (b) Calculate the pH of a 0.265 M solution of propanoic acid. (c) A 0.496 g sample of sodium propanoate, NaC3H5O2 , is added to a 50.0 mL sample of a 0.265 M solution of propanoic acid. Assuming that no change in the volume of the solut ...
... (a) Write the equilibrium-constant expression for the reaction. (b) Calculate the pH of a 0.265 M solution of propanoic acid. (c) A 0.496 g sample of sodium propanoate, NaC3H5O2 , is added to a 50.0 mL sample of a 0.265 M solution of propanoic acid. Assuming that no change in the volume of the solut ...