PURPOSE: To determine the value of the equilibrium constant for a
... 8. An error was made in preparing the KSCN solution in Part A. Its concentration was 0.003 molar but was labeled as 0.002 molar. How would the slope of the calibration curve (absorbance on the y-axis versus concentration of Fe(SCN)2+on the x-axis) be affected? How would this impact your Kc in Part C ...
... 8. An error was made in preparing the KSCN solution in Part A. Its concentration was 0.003 molar but was labeled as 0.002 molar. How would the slope of the calibration curve (absorbance on the y-axis versus concentration of Fe(SCN)2+on the x-axis) be affected? How would this impact your Kc in Part C ...
Normality Primer
... 10. A 0.9932 g sample of limestone was titrated with 15.67 mL of 0.113 N HCl, what is the percent of calcium carbonate in the sample? 11. 27.44 mL of 0.222 N Ba(OH)2 was required to neutralize all the benzoic acid (C6H5COOH) in a 1.224 g sample of organic material. What was the percent benzo ...
... 10. A 0.9932 g sample of limestone was titrated with 15.67 mL of 0.113 N HCl, what is the percent of calcium carbonate in the sample? 11. 27.44 mL of 0.222 N Ba(OH)2 was required to neutralize all the benzoic acid (C6H5COOH) in a 1.224 g sample of organic material. What was the percent benzo ...
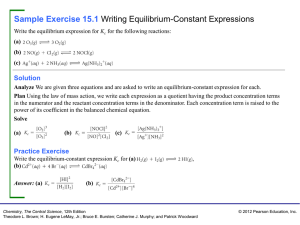
Sample Exercise 15.1 Writing Equilibrium
... Analyze We are asked which of several combinations of species can establish an equilibrium between calcium carbonate and its decomposition products, calcium oxide and carbon dioxide. Plan For equilibrium to be achieved, it must be possible for both the forward process and the reverse process to occu ...
... Analyze We are asked which of several combinations of species can establish an equilibrium between calcium carbonate and its decomposition products, calcium oxide and carbon dioxide. Plan For equilibrium to be achieved, it must be possible for both the forward process and the reverse process to occu ...
Stoichiometry, Lab Basics, Reactions
... CaCl2. What is the minimum number of moles of AgNO3 that must be added to the solution in order to precipitate all of the Cl- as AgCl (s)? (Assume all AgCl is insoluble.) A) 0.10 mol B) 0.20 mol C) 0.30 mol D) 0.40 mol E) 0.60 mol ____ 21. A 40.0 mL sample of 0.25 M KOH is added to 60.0 mL of 0.15 M ...
... CaCl2. What is the minimum number of moles of AgNO3 that must be added to the solution in order to precipitate all of the Cl- as AgCl (s)? (Assume all AgCl is insoluble.) A) 0.10 mol B) 0.20 mol C) 0.30 mol D) 0.40 mol E) 0.60 mol ____ 21. A 40.0 mL sample of 0.25 M KOH is added to 60.0 mL of 0.15 M ...
Chapter 17: Reaction Energy and Reaction Kinetics
... always interpret the coefficients as numbers of moles and never as numbers of molecules. The quantity of energy released as heat in this or any reaction depends on the amounts of reactants and products. The quantity of energy released during the formation of water from H2 and O2 is proportional to t ...
... always interpret the coefficients as numbers of moles and never as numbers of molecules. The quantity of energy released as heat in this or any reaction depends on the amounts of reactants and products. The quantity of energy released during the formation of water from H2 and O2 is proportional to t ...
Chemical Reactions
... You prepared cookie dough to make 5 dozen cookies. The phone rings while a sheet of 12 cookies is baking. You talk too long and the cookies burn. You throw them out (or give them to your dog.) The rest of the cookies are okay. How many cookies could you have made (theoretical ...
... You prepared cookie dough to make 5 dozen cookies. The phone rings while a sheet of 12 cookies is baking. You talk too long and the cookies burn. You throw them out (or give them to your dog.) The rest of the cookies are okay. How many cookies could you have made (theoretical ...
Chapter 3: Calculations with Chemical Formulas
... 2H+(aq) + 2NO3−(aq) + Mg(OH)2(s) 2H2O(l) + Mg2+(aq) + 2NO3−(aq) The corresponding net ionic equation is: 2H+(aq) + Mg(OH)2(s) 2H2O(l) + Mg2+(aq) The resulting complete ionic equation is: Pb2+(aq) + 2NO3−(aq) + 2Na+(aq) + SO42−(aq) PbSO4(s) + 2Na+(aq) + 2NO3−(aq) The corresponding net ionic equ ...
... 2H+(aq) + 2NO3−(aq) + Mg(OH)2(s) 2H2O(l) + Mg2+(aq) + 2NO3−(aq) The corresponding net ionic equation is: 2H+(aq) + Mg(OH)2(s) 2H2O(l) + Mg2+(aq) The resulting complete ionic equation is: Pb2+(aq) + 2NO3−(aq) + 2Na+(aq) + SO42−(aq) PbSO4(s) + 2Na+(aq) + 2NO3−(aq) The corresponding net ionic equ ...
CHEMICAL REACTIONS
... • Take one element at a time usually starting with the most complex substance. • It is usually better to balance in this order: metals, nonmetals, hydrogen, oxygen. • If everything balances except for O2, and there is no way to balance O2 with a whole number, use a fraction or mixed number. Then, mu ...
... • Take one element at a time usually starting with the most complex substance. • It is usually better to balance in this order: metals, nonmetals, hydrogen, oxygen. • If everything balances except for O2, and there is no way to balance O2 with a whole number, use a fraction or mixed number. Then, mu ...
Chapter 16.1
... Heat and Temperature • The energy absorbed or released as heat in a chemical or physical change is measured in a calorimeter. • In one kind of calorimeter, known quantities of reactants are sealed in a reaction chamber that is immersed in a known quantity of water. • Energy given off by the reaction ...
... Heat and Temperature • The energy absorbed or released as heat in a chemical or physical change is measured in a calorimeter. • In one kind of calorimeter, known quantities of reactants are sealed in a reaction chamber that is immersed in a known quantity of water. • Energy given off by the reaction ...
03_Worked_Examples
... (c) The reactants box contains four O2 and eight NO. Thus, the molecular ratio is one O2 for each two NO, as required by the balanced equation. The products box contains eight NO 2, which means the number of NO2 product molecules equals the number of NO reactant molecules, as the balanced equation r ...
... (c) The reactants box contains four O2 and eight NO. Thus, the molecular ratio is one O2 for each two NO, as required by the balanced equation. The products box contains eight NO 2, which means the number of NO2 product molecules equals the number of NO reactant molecules, as the balanced equation r ...
The first practical method for asymmetric epoxidation
... Analysis of this material as the MTPA ester” gave an enantiomeric excess (ee) of >95% whereas analysis of the derived epoxy acetate by using Eu(hfbc)3 chiral shift reagent gave 94% ee. The “typical procedure” given for geraniol has a limitation which is important to emphasize. Very poor yields are r ...
... Analysis of this material as the MTPA ester” gave an enantiomeric excess (ee) of >95% whereas analysis of the derived epoxy acetate by using Eu(hfbc)3 chiral shift reagent gave 94% ee. The “typical procedure” given for geraniol has a limitation which is important to emphasize. Very poor yields are r ...
N5 Chemistry Course Specification 2017-18 session
... Isotopes are defined as atoms with the same atomic number but different mass numbers, or as atoms with the same number of protons but different numbers of neutrons. Nuclide notation is used to show the atomic number, mass number (and charge) of atoms (ions) from which the number of protons, electron ...
... Isotopes are defined as atoms with the same atomic number but different mass numbers, or as atoms with the same number of protons but different numbers of neutrons. Nuclide notation is used to show the atomic number, mass number (and charge) of atoms (ions) from which the number of protons, electron ...
Physical Chemistry 3: — Chemical Kinetics - Christian
... Figure 1.1: Observed concentration-time profile of sucrose. ...
... Figure 1.1: Observed concentration-time profile of sucrose. ...
Unit 6- Math of Chemistry
... • What is the percent composition of O in KClO3? • 1st: Determine formula mass of element and of the compound • 2nd: % of element = element formula mass/ total formula mass x 100 ...
... • What is the percent composition of O in KClO3? • 1st: Determine formula mass of element and of the compound • 2nd: % of element = element formula mass/ total formula mass x 100 ...
2013-2014
... Answers to Section A should be marked on the Multiple-choice Answer Sheet while answers to Section B should be written in the spaces provided in Question-Answer Book B. The Answer Sheet for Section A and the Question-Answer Book for Section B will be collected separately at the end of the examinatio ...
... Answers to Section A should be marked on the Multiple-choice Answer Sheet while answers to Section B should be written in the spaces provided in Question-Answer Book B. The Answer Sheet for Section A and the Question-Answer Book for Section B will be collected separately at the end of the examinatio ...