Part V The Third Law and Free Energy
... crystals of different types of atom is associated with an increase in entropy. A solid solution will not have a positive entropy unless it possesses some degree of randomness. If each atom of one type bears a definite spatial relation to atoms of the other kind, the system will have a low thermodyna ...
... crystals of different types of atom is associated with an increase in entropy. A solid solution will not have a positive entropy unless it possesses some degree of randomness. If each atom of one type bears a definite spatial relation to atoms of the other kind, the system will have a low thermodyna ...
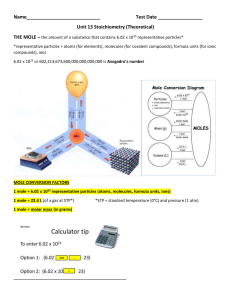
Unit 13 Stoichiometry (Theoretical)
... b. How many grams of acetylene (C2H2) are produced by adding water to 5.00 grams of CaC2? (ans. 2.03 g C2H2) ...
... b. How many grams of acetylene (C2H2) are produced by adding water to 5.00 grams of CaC2? (ans. 2.03 g C2H2) ...
Chap 3 - HCC Learning Web
... 1 in front of it to remind us we have done examining C4H10. Now the equation is updated to be 1 C4H10 + __ O2 __ CO2 + __ H2O Since C4H10 contains 4 carbon atoms, so we need four carbon atoms at the right side, which leads us to put 4 (called coefficient) in front of the CO2. Now the equation is u ...
... 1 in front of it to remind us we have done examining C4H10. Now the equation is updated to be 1 C4H10 + __ O2 __ CO2 + __ H2O Since C4H10 contains 4 carbon atoms, so we need four carbon atoms at the right side, which leads us to put 4 (called coefficient) in front of the CO2. Now the equation is u ...
Chap 3 - HCC Learning Web
... 1 in front of it to remind us we have done examining C4H10. Now the equation is updated to be 1 C4H10 + __ O2 __ CO2 + __ H2O Since C4H10 contains 4 carbon atoms, so we need four carbon atoms at the right side, which leads us to put 4 (called coefficient) in front of the CO2. Now the equation is u ...
... 1 in front of it to remind us we have done examining C4H10. Now the equation is updated to be 1 C4H10 + __ O2 __ CO2 + __ H2O Since C4H10 contains 4 carbon atoms, so we need four carbon atoms at the right side, which leads us to put 4 (called coefficient) in front of the CO2. Now the equation is u ...
Microwave-Specific Enhancement of the Carbon−Carbon Dioxide
... comes up to temperature, the flow of CO2 commenced using the mass flow controller. As with microwave experiments, the volume of gas evolved during the reaction was measured using a totalizing mass flow meter. The product gas was sampled at the same 1 min intervals as in the microwave experiment and ana ...
... comes up to temperature, the flow of CO2 commenced using the mass flow controller. As with microwave experiments, the volume of gas evolved during the reaction was measured using a totalizing mass flow meter. The product gas was sampled at the same 1 min intervals as in the microwave experiment and ana ...
Chapter 4 – Reactions in Aqueous Solutions
... – To obtain the overall equation, we add the two balanced half-equations, but make sure the number of electrons on both half-equations are equal, so that they cancel out. The overall equation should not contain any electrons. In this case, we multiply the above oxidation half-equation by 5 and obtai ...
... – To obtain the overall equation, we add the two balanced half-equations, but make sure the number of electrons on both half-equations are equal, so that they cancel out. The overall equation should not contain any electrons. In this case, we multiply the above oxidation half-equation by 5 and obtai ...
Thermochemistry Exam Review Questions
... B. Cl- ions are repelled by the hydrogen atoms of the water molecules C. Na+ ions are attracted to the oxygen atoms of the water molecules D. Na+ ions are repelled by the oxygen atoms of the water molecules 13 Which of the following would produce a precipitate when equal volumes of 0.5 mol/L of aqu ...
... B. Cl- ions are repelled by the hydrogen atoms of the water molecules C. Na+ ions are attracted to the oxygen atoms of the water molecules D. Na+ ions are repelled by the oxygen atoms of the water molecules 13 Which of the following would produce a precipitate when equal volumes of 0.5 mol/L of aqu ...
The Process of Chemical Reactions
... develop ways to make chemical products more efficiently, more safely, and more economically. This chapter introduces a model for visualizing the changes that take place in a reaction mixture as a chemical reaction proceeds. The model describes the requirements that must be met before a reaction can ...
... develop ways to make chemical products more efficiently, more safely, and more economically. This chapter introduces a model for visualizing the changes that take place in a reaction mixture as a chemical reaction proceeds. The model describes the requirements that must be met before a reaction can ...
coordination compounds - Ahlcon Public School , Mayur Vihar Ph
... The monomers of starch is - glucose and that of cellulose is - glucose. The only difference is in the position of H and OH is at carbon one. ...
... The monomers of starch is - glucose and that of cellulose is - glucose. The only difference is in the position of H and OH is at carbon one. ...
Topic 3: Chemical Kinetics - Manitoba Education and Training
... Ask students for examples of fast and slow reactions or processes that they encounter in their daily lives. Students may begin with examples of physical changes, such as melting or dissolving. Even though these are not examples of chemical changes, they still reinforce the concept of fast and slow r ...
... Ask students for examples of fast and slow reactions or processes that they encounter in their daily lives. Students may begin with examples of physical changes, such as melting or dissolving. Even though these are not examples of chemical changes, they still reinforce the concept of fast and slow r ...
CARBANIONS Carbanions are units that contain a negative charge
... Enamines, the products of the acid-catalyzed addition of secondary amines to aldehydes or ketones, can be viewed as weakly nucleophilic enolate anions. Enamines will react with a,b-unsaturated carbonyl systems in the Michael reaction in method that permits introduction of new carbon-carbon bonds adj ...
... Enamines, the products of the acid-catalyzed addition of secondary amines to aldehydes or ketones, can be viewed as weakly nucleophilic enolate anions. Enamines will react with a,b-unsaturated carbonyl systems in the Michael reaction in method that permits introduction of new carbon-carbon bonds adj ...
Objective (Local, State, National – College Board)
... reactants and products will remain the same regardless of how much material is in the central beaker. Introduce calculation of Kc and Kp. Use laboratory work to reinforce concepts. Introduce Q, the reaction quotient, a measure of how close a system is to equilibrium. Use the see saw analogy to expla ...
... reactants and products will remain the same regardless of how much material is in the central beaker. Introduce calculation of Kc and Kp. Use laboratory work to reinforce concepts. Introduce Q, the reaction quotient, a measure of how close a system is to equilibrium. Use the see saw analogy to expla ...
SOL Review Part 3 Nomenclature reactions
... When naming a transition metal that has more than one oxidation number, the numeric value of the oxidation number is indicated by a — A Roman numeral _ B Greek prefix C subscript D suffix ...
... When naming a transition metal that has more than one oxidation number, the numeric value of the oxidation number is indicated by a — A Roman numeral _ B Greek prefix C subscript D suffix ...
Unit 3 Exam Level Questions
... (b) A sample of oceanic water was found to contain 0·010 g of dissolved oxygen. Calculate the number of moles of dissolved oxygen present in the sample. ...
... (b) A sample of oceanic water was found to contain 0·010 g of dissolved oxygen. Calculate the number of moles of dissolved oxygen present in the sample. ...
CIS Exam Questions
... C The mass of water formed D The mass of copper(II) carbonate dissolved 29. Which of the following is the best description of a feedstock? A A consumer product such as a textile, plastic or detergent. B A complex chemical that has been synthesised from small molecules. C A mixture of chemicals forme ...
... C The mass of water formed D The mass of copper(II) carbonate dissolved 29. Which of the following is the best description of a feedstock? A A consumer product such as a textile, plastic or detergent. B A complex chemical that has been synthesised from small molecules. C A mixture of chemicals forme ...
Stoichiometry and the Mole
... Limiting Reactants The limiting reactant (or limiting reagent) is the reactant that is entirely consumed when a reaction goes to completion. The moles of product are always determined by the starting moles of the limiting reactant. ...
... Limiting Reactants The limiting reactant (or limiting reagent) is the reactant that is entirely consumed when a reaction goes to completion. The moles of product are always determined by the starting moles of the limiting reactant. ...
PURPOSE: To determine the value of the equilibrium constant for a
... 8. An error was made in preparing the KSCN solution in Part A. Its concentration was 0.003 molar but was labeled as 0.002 molar. How would the slope of the calibration curve (absorbance on the y-axis versus concentration of Fe(SCN)2+on the x-axis) be affected? How would this impact your Kc in Part C ...
... 8. An error was made in preparing the KSCN solution in Part A. Its concentration was 0.003 molar but was labeled as 0.002 molar. How would the slope of the calibration curve (absorbance on the y-axis versus concentration of Fe(SCN)2+on the x-axis) be affected? How would this impact your Kc in Part C ...