Oxidation-Reduction Reactions
... normal balancing it is a required step in the process of redox reactions. One of the most accepted methods of balancing a redox reaction is known as the half-equation method, however it can become more complex when involving basic or acidic solutions. In this module, a brief introduction to this dif ...
... normal balancing it is a required step in the process of redox reactions. One of the most accepted methods of balancing a redox reaction is known as the half-equation method, however it can become more complex when involving basic or acidic solutions. In this module, a brief introduction to this dif ...
CHEM 1212 Module Ten-Chapter 16 Name
... contributes to air pollution whenever fuel is burned at high temperatures and high pressure in an automobile gasoline engine. At 1500 K, K= 1.0 x 10 -5. Suppose a sample of air has [N2] = 0.80 M and [O2] = 0.20 M before any reaction occurs. Calculate the equilibrium concentrations of the reactants a ...
... contributes to air pollution whenever fuel is burned at high temperatures and high pressure in an automobile gasoline engine. At 1500 K, K= 1.0 x 10 -5. Suppose a sample of air has [N2] = 0.80 M and [O2] = 0.20 M before any reaction occurs. Calculate the equilibrium concentrations of the reactants a ...
Audit Schedule
... 1. It is imperative that you learn how to convert moles to grams and grams to moles. This simple conversion is a basic step in a great many problems that will be encountered throughout chemistry. 2. Remember that a single chemical symbol can have several different meanings. For instance, Fe can mean ...
... 1. It is imperative that you learn how to convert moles to grams and grams to moles. This simple conversion is a basic step in a great many problems that will be encountered throughout chemistry. 2. Remember that a single chemical symbol can have several different meanings. For instance, Fe can mean ...
Stoichiometry File
... When gasoline is burned in an engine, all of these various compounds undergo combustion simultaneously, reacting with oxygen from the air. As you might imagine, an accurate description of the combustion of such a complex mixture of compounds would be quite a challenge. So for our present purposes, i ...
... When gasoline is burned in an engine, all of these various compounds undergo combustion simultaneously, reacting with oxygen from the air. As you might imagine, an accurate description of the combustion of such a complex mixture of compounds would be quite a challenge. So for our present purposes, i ...
Modeling the Rate of Heterogeneous Reactions
... one lattice type and cannot represent the different neighborhoods of combinations like fcc(111) and fcc(100)-faces. A hybrid approach between a lattice and off-lattice method can overcome these limitations. The facets of the catalyst particle and the support are each described by a lattice, which ar ...
... one lattice type and cannot represent the different neighborhoods of combinations like fcc(111) and fcc(100)-faces. A hybrid approach between a lattice and off-lattice method can overcome these limitations. The facets of the catalyst particle and the support are each described by a lattice, which ar ...
Questions - Scheikundeolympiade
... QUESTION 28: Cryoscopy (4 points) Chemists often need a bath in which to carry out a process that has a temperature below the water freezing point (0 °C) and well above the CO2 sublimation point (78 °C). In this case they mix water ice prepared at its melting point and NaCl. Depending on the quanti ...
... QUESTION 28: Cryoscopy (4 points) Chemists often need a bath in which to carry out a process that has a temperature below the water freezing point (0 °C) and well above the CO2 sublimation point (78 °C). In this case they mix water ice prepared at its melting point and NaCl. Depending on the quanti ...
Read Sections 3.3. Avogadro`s Number and the Mole
... present in a given mass or number of moles of a compound. Learning Objective 15: Use Avogadro's number to convert between the number of moles (or mass) and number of molecules (or atoms). Do Problems 7 - 8 at the end of the section Do the following end-of-chapter problems: 31, 58, 60, 62, 64 ...
... present in a given mass or number of moles of a compound. Learning Objective 15: Use Avogadro's number to convert between the number of moles (or mass) and number of molecules (or atoms). Do Problems 7 - 8 at the end of the section Do the following end-of-chapter problems: 31, 58, 60, 62, 64 ...
Electrochemical Fundamentals
... British physical chemist who was the first to connect the kinetic electrochemistry built up in the second half of the twentieth century with the thermodynamic electrochemistry that dominated the first half. He had to his credit, not only the first exponential relation between current and potential ( ...
... British physical chemist who was the first to connect the kinetic electrochemistry built up in the second half of the twentieth century with the thermodynamic electrochemistry that dominated the first half. He had to his credit, not only the first exponential relation between current and potential ( ...
Chap 3 - HCC Learning Web
... 1 in front of it to remind us we have done examining C4H10. Now the equation is updated to be 1 C4H10 + O2 CO2 + H2O Since C4H10 contains 4 carbon atoms, so we need four carbon atoms at the right side, which leads us to put 4 (called coefficient) in front of the CO2. Now the equation is updated to ...
... 1 in front of it to remind us we have done examining C4H10. Now the equation is updated to be 1 C4H10 + O2 CO2 + H2O Since C4H10 contains 4 carbon atoms, so we need four carbon atoms at the right side, which leads us to put 4 (called coefficient) in front of the CO2. Now the equation is updated to ...
Role of Chemical Reaction Engineering in Sustainable
... they are highly corrosive and toxic. Therefore, based on environmental concerns these catalysts are now being replaced by solid-acid catalysts. Once selected, to further tailor the catalyst to get optimum yield and selectivity, physical properties of the catalyst have to be determined and improved. ...
... they are highly corrosive and toxic. Therefore, based on environmental concerns these catalysts are now being replaced by solid-acid catalysts. Once selected, to further tailor the catalyst to get optimum yield and selectivity, physical properties of the catalyst have to be determined and improved. ...
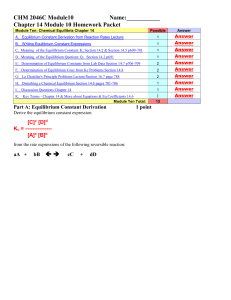
Chemistry 30 - SharpSchool
... ___________________________________________ which says that there is a ________________________________ between the concentrations of the products and the concentrations of the reactants at equilibrium ...
... ___________________________________________ which says that there is a ________________________________ between the concentrations of the products and the concentrations of the reactants at equilibrium ...
2. The Magic of Chemical Reactions
... orange because of ------. Chemical reaction involves breaking and making of the bonds between the atoms to produce ------ ------. The chemical reaction during which H2(g) is lost is termed as ------. When oil and fats are oxidised or even allowed to stand for a long time, they become ------. The che ...
... orange because of ------. Chemical reaction involves breaking and making of the bonds between the atoms to produce ------ ------. The chemical reaction during which H2(g) is lost is termed as ------. When oil and fats are oxidised or even allowed to stand for a long time, they become ------. The che ...
Evidence for the Predominance of Condensed Phase Reaction in
... This was further investigated using the temperature-jump/timeof-flight mass spectrometer (T-Jump/TOFMS) and a C/Bi2O3 mixture, for which a condensed phase reaction was also observed. However, Bi2O3 is a unique oxidizer in that it changes crystalline phase to δ-Bi2O3 shortly before melting and becomes ...
... This was further investigated using the temperature-jump/timeof-flight mass spectrometer (T-Jump/TOFMS) and a C/Bi2O3 mixture, for which a condensed phase reaction was also observed. However, Bi2O3 is a unique oxidizer in that it changes crystalline phase to δ-Bi2O3 shortly before melting and becomes ...
SCH4U Exam Review
... (c) 2Fe(s) + O2 (g) 2FeO(s) (d) SO2 (g) + ½O2 (g) SO3 (g) ANS: (b) and (c) 9.Use the standard enthalpies of formation (∆Hf) table to calculate ∆H for the following reactions: (a) CaO(s) + CO2 (g) CaCO3 (s) ANS: ∆H = -178.61 kJ (b) C2H2 (g) + 2H2 (g) C2H6 (g) ...
... (c) 2Fe(s) + O2 (g) 2FeO(s) (d) SO2 (g) + ½O2 (g) SO3 (g) ANS: (b) and (c) 9.Use the standard enthalpies of formation (∆Hf) table to calculate ∆H for the following reactions: (a) CaO(s) + CO2 (g) CaCO3 (s) ANS: ∆H = -178.61 kJ (b) C2H2 (g) + 2H2 (g) C2H6 (g) ...
CHEMICAL EQUILIBRIUM
... CALCULATING EQUILIBRIUM PRESSURES AND CONCENTRATIONS ***** Dinitrogen tetroxide in its liquid state was used as one of the fuels on the lunar lander for the NASA Apollo missions. In the gas phase it decomposes to gaseous nitrogen dioxide: N2O4 (g) ↔ 2 NO2 (g) Consider an experiment in which gaseous ...
... CALCULATING EQUILIBRIUM PRESSURES AND CONCENTRATIONS ***** Dinitrogen tetroxide in its liquid state was used as one of the fuels on the lunar lander for the NASA Apollo missions. In the gas phase it decomposes to gaseous nitrogen dioxide: N2O4 (g) ↔ 2 NO2 (g) Consider an experiment in which gaseous ...
IX Chemistry Chapter 02
... By fixing the mass of (N), the mass of (O) in different oxides varies. i.e. 8 : 16 : 24 : 32 : 40 ...
... By fixing the mass of (N), the mass of (O) in different oxides varies. i.e. 8 : 16 : 24 : 32 : 40 ...
Ch 10 - Enrico Fermi High School
... In an experiment involving the determination of the equilibrium constant for a reaction, 10.0 mL of 2.00 x 10-3 M Fe+3 (aq) was mixed with 20.0 mL of 4.00 x 10-3 M SCN-(aq). The number of moles of FeSCN2+(aq) that was formed after the reaction of Fe+3 (aq) and SCN-(aq) came to equilibrium was 3.50 x ...
... In an experiment involving the determination of the equilibrium constant for a reaction, 10.0 mL of 2.00 x 10-3 M Fe+3 (aq) was mixed with 20.0 mL of 4.00 x 10-3 M SCN-(aq). The number of moles of FeSCN2+(aq) that was formed after the reaction of Fe+3 (aq) and SCN-(aq) came to equilibrium was 3.50 x ...
Chemistry - Onslow College
... Names and formula of common lab acids and bases Reactions of acids with metals, bases and metal carbonates Acids, bases and indicators eg litmus, methyl orange and phenol phthalein; UI pH scale salts formed from reaction of acid with metal, base or carbonate By the end of this topic studen ...
... Names and formula of common lab acids and bases Reactions of acids with metals, bases and metal carbonates Acids, bases and indicators eg litmus, methyl orange and phenol phthalein; UI pH scale salts formed from reaction of acid with metal, base or carbonate By the end of this topic studen ...
Word - chemmybear.com
... 11. Ammonium chloride is placed inside a closed vessel where it comes into equilibrium at 400C according to the equation shown. Only these three substances are present inside the vessel. If Kp for the system at 400C is 0.640, what is the pressure inside the vessel? NH4Cl(s) NH3(g) + HCl(g) 12. Bro ...
... 11. Ammonium chloride is placed inside a closed vessel where it comes into equilibrium at 400C according to the equation shown. Only these three substances are present inside the vessel. If Kp for the system at 400C is 0.640, what is the pressure inside the vessel? NH4Cl(s) NH3(g) + HCl(g) 12. Bro ...