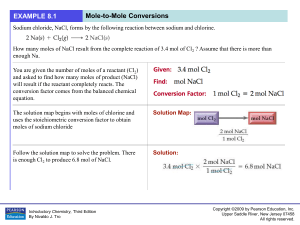
Untitled
... dioxide and water. If 62.0 g of CO2 is produced when 22.5 g of C2H2 reacts with sufficient oxygen, what is the percent yield of CO2 for the reaction? (1, 6, 7, 8) When 50.0 g of iron(III) oxide reacts with carbon monoxide, 32.8 g of iron is produced. What is the percent yield of Fe for the reaction? ...
... dioxide and water. If 62.0 g of CO2 is produced when 22.5 g of C2H2 reacts with sufficient oxygen, what is the percent yield of CO2 for the reaction? (1, 6, 7, 8) When 50.0 g of iron(III) oxide reacts with carbon monoxide, 32.8 g of iron is produced. What is the percent yield of Fe for the reaction? ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... Adding this coefficient balances H but gives four O atoms in the products. Because there are only three O atoms in the reactants, we are not finished. We can place the 3/2 coefficient in front of O2 to give four O Atoms in the reactants (3/2 x 2 = 3 O atoms in 3/2 O2) CH3OH(l) + 3/2O2(g) CO2(g) + ...
... Adding this coefficient balances H but gives four O atoms in the products. Because there are only three O atoms in the reactants, we are not finished. We can place the 3/2 coefficient in front of O2 to give four O Atoms in the reactants (3/2 x 2 = 3 O atoms in 3/2 O2) CH3OH(l) + 3/2O2(g) CO2(g) + ...
Activation of Alcohols Toward Nucleophilic Substitution: Conversion
... alcohols are converted to saturated alkyl halides.6 Because the use of HCl shows poor results for the conversion of an alcohol to an alkyl chloride, a catalyst such as the zinc used in the Lucas reagent is required. This reaction was improved by adding zinc chloride and had the advantage of milder c ...
... alcohols are converted to saturated alkyl halides.6 Because the use of HCl shows poor results for the conversion of an alcohol to an alkyl chloride, a catalyst such as the zinc used in the Lucas reagent is required. This reaction was improved by adding zinc chloride and had the advantage of milder c ...
2015 chemistry
... Explain why the percentage of oil converted in an enzyme-catalysed reaction is very low at high temperatures. _______________________________________________________________________________________________________ ______________________________________________________________________________________ ...
... Explain why the percentage of oil converted in an enzyme-catalysed reaction is very low at high temperatures. _______________________________________________________________________________________________________ ______________________________________________________________________________________ ...
ANSWERS Problem Set 5a – Chemical Reactions
... 34) Define “Reactant in Excess” and “Limiting Reactant.” Why are these two terms important in industrial production of compounds? The reactant is excess is the substance that remains after the limiting reactant runs out. The limiting reactant is the reactant that runs out before any other in a chemi ...
... 34) Define “Reactant in Excess” and “Limiting Reactant.” Why are these two terms important in industrial production of compounds? The reactant is excess is the substance that remains after the limiting reactant runs out. The limiting reactant is the reactant that runs out before any other in a chemi ...
Solved Examples
... In photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H12O6) according to the following reaction. How many grams of glucose can be synthesized from 58.5 g of CO2 ? Assume that there is more than enough water present to react with all of the CO2 . Set up the problem in the norm ...
... In photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H12O6) according to the following reaction. How many grams of glucose can be synthesized from 58.5 g of CO2 ? Assume that there is more than enough water present to react with all of the CO2 . Set up the problem in the norm ...
Stoichiometry - HCC Learning Web
... • Combustion reactions are generally rapid reactions that produce a flame. • Combustion reactions most often involve oxygen in the air as a reactant. ...
... • Combustion reactions are generally rapid reactions that produce a flame. • Combustion reactions most often involve oxygen in the air as a reactant. ...
Chemical Reaction
... A chemical reaction involves the rearrangement of atoms. produces one or more new substances. can be observed by the appearance of new physical properties. A chemical reaction forms new products with different properties. An antacid (NaHCO3) tablet in water forms bubbles of carbon dioxide (CO2 ...
... A chemical reaction involves the rearrangement of atoms. produces one or more new substances. can be observed by the appearance of new physical properties. A chemical reaction forms new products with different properties. An antacid (NaHCO3) tablet in water forms bubbles of carbon dioxide (CO2 ...
Worksheet Significant Figures
... graphs are used when the data is qualitative (descriptive, based on observations or categories of data). Line graphs are used when the data is quantitative (more precise, measured with tools). **VERY IMPORTANT** When designing an experiment, you should have only one independent and one dependent var ...
... graphs are used when the data is qualitative (descriptive, based on observations or categories of data). Line graphs are used when the data is quantitative (more precise, measured with tools). **VERY IMPORTANT** When designing an experiment, you should have only one independent and one dependent var ...
Chemical Reactions and Equations - 2012 Book Archive
... Unfortunately, it is also an incomplete chemical equation. The law of conservation of matter says that matter cannot be created or destroyed. In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. If we cou ...
... Unfortunately, it is also an incomplete chemical equation. The law of conservation of matter says that matter cannot be created or destroyed. In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. If we cou ...
Basic Stoichometry
... Imagine if you made a batch of cookies and used way too many eggs, or not enough sugar. YUCK! In chemistry, reactions proceed with very specific recipes. The study of these recipes is stoichiometry. When the reactants are present in the correct amounts, the reaction will produce products. What happe ...
... Imagine if you made a batch of cookies and used way too many eggs, or not enough sugar. YUCK! In chemistry, reactions proceed with very specific recipes. The study of these recipes is stoichiometry. When the reactants are present in the correct amounts, the reaction will produce products. What happe ...
Chem101 - Lecture 5 Introduction Introduction
... • In this reaction the sulfur is oxidized while the oxygen is reduced. University of Wisconsin-Eau Claire ...
... • In this reaction the sulfur is oxidized while the oxygen is reduced. University of Wisconsin-Eau Claire ...
electrical energy and capacitance
... Example 1. A compound is discovered with a 58.12 g/mol molar mass. Its empirical formula is C2H5. What is the molecular formula of this compound? 1A. (1) C = 12.01 amu (2) H = 1.01 amu (3) C2 + H5 (4) C2H5 = 2(12.01 amu) + 5(1.01 amu) (5) EF = C2H5 = 29.07 g/mol (6) MF = 58.12 g/mol (7) MF = n(EF) ( ...
... Example 1. A compound is discovered with a 58.12 g/mol molar mass. Its empirical formula is C2H5. What is the molecular formula of this compound? 1A. (1) C = 12.01 amu (2) H = 1.01 amu (3) C2 + H5 (4) C2H5 = 2(12.01 amu) + 5(1.01 amu) (5) EF = C2H5 = 29.07 g/mol (6) MF = 58.12 g/mol (7) MF = n(EF) ( ...
введение в общую introductio to the general ch ведение в общую
... The question from the title of this subsection is very important. It can be rephrased in the following ways. What is chemistry? What is the subject of the discipline you are starting (or, hopefully, continuing) to study? What is the difference between chemistry and physics? Physical properties of a ...
... The question from the title of this subsection is very important. It can be rephrased in the following ways. What is chemistry? What is the subject of the discipline you are starting (or, hopefully, continuing) to study? What is the difference between chemistry and physics? Physical properties of a ...
AP Chemistry
... C3H8 burns in excess oxygen gas. What is the coefficient for O2 when the equation is balanced with lowest terms? ...
... C3H8 burns in excess oxygen gas. What is the coefficient for O2 when the equation is balanced with lowest terms? ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • 2Mg(s) + O2(g) 2MgO(s) Since there are fewer products than reactants, the Mg has combined with O2 to form MgO. 2. Note that the structure of the reactants has changed. 3. Mg consists of closely packed atoms and O2 consists of dispersed molecules. 4. MgO consists of a lattice of Mg2+ and O2– ions ...
... • 2Mg(s) + O2(g) 2MgO(s) Since there are fewer products than reactants, the Mg has combined with O2 to form MgO. 2. Note that the structure of the reactants has changed. 3. Mg consists of closely packed atoms and O2 consists of dispersed molecules. 4. MgO consists of a lattice of Mg2+ and O2– ions ...