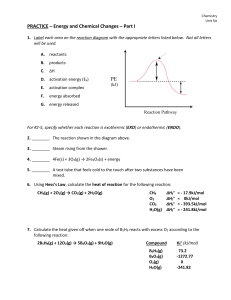
Learning Outcomes for Chemical Reactions and
... all bases that can neutralise an acid. 13. A salt is formed whenever an acid is neutralised. 14. A titration is used to find the unknown concentration of an acid or an alkali. 15. By identifying spectator ions and removing (omitting) them from a reaction equation, you can state what the reacting ion ...
... all bases that can neutralise an acid. 13. A salt is formed whenever an acid is neutralised. 14. A titration is used to find the unknown concentration of an acid or an alkali. 15. By identifying spectator ions and removing (omitting) them from a reaction equation, you can state what the reacting ion ...
Chem 30A Final Exam
... 1. Draw valid Lewis structures for the simplest compounds of the second row elements (except for Li) with flourine including lone pair electrons. Indicate the valence (i.e. # of bonds), the central atom geometry, and the approximate bond angles in each case. Also indicate when there is an exception ...
... 1. Draw valid Lewis structures for the simplest compounds of the second row elements (except for Li) with flourine including lone pair electrons. Indicate the valence (i.e. # of bonds), the central atom geometry, and the approximate bond angles in each case. Also indicate when there is an exception ...
Chapter 3
... 1 molecule N2 = 3 molecules H2 1 molecule N2 = 2 molecules NH3 3 molecules H2 = 2 molecules NH3 LEP #7 ...
... 1 molecule N2 = 3 molecules H2 1 molecule N2 = 2 molecules NH3 3 molecules H2 = 2 molecules NH3 LEP #7 ...
1) In the reaction H2O + CH3COOH H3O+ + CH3COO
... 7) (20 points) Let's say that a galvanic cell (spontaneous oxidation/reduction reaction like a battery) is constructed using the half reactions Cu2+(aq) + 2e- Cu(s) (E0 = +0.34V) and Zn2+ + 2e- Zn(s) (E0 = -0.76). What ratio of concentrations, Zn2+/Cu2+, in the electrolyte will be required if o ...
... 7) (20 points) Let's say that a galvanic cell (spontaneous oxidation/reduction reaction like a battery) is constructed using the half reactions Cu2+(aq) + 2e- Cu(s) (E0 = +0.34V) and Zn2+ + 2e- Zn(s) (E0 = -0.76). What ratio of concentrations, Zn2+/Cu2+, in the electrolyte will be required if o ...
Erik`s Chemistry: Thermochemistry - ECHS Chemistry
... calculate the H in kJ if 5.8 grams of oxygen are consumed in the process. = 81 kJ H= -81kJ B. Ammonium nitrate, NH4NO3, is commonly used as an explosive. It decomposes by the following reaction: NH4NO3 ...
... calculate the H in kJ if 5.8 grams of oxygen are consumed in the process. = 81 kJ H= -81kJ B. Ammonium nitrate, NH4NO3, is commonly used as an explosive. It decomposes by the following reaction: NH4NO3 ...
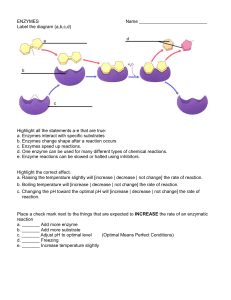
ENZYMES
... a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
... a. _______ Add more enzyme b. _______ Add more substrate c. _______ Adjust pH to optimal level (Optimal Means Perfect Conditions) d. _______ Freezing e. _______ Increase temperature slightly ...
Chapter 11
... silver chloride reacts with aqueous sodium bromide to produce aqueous silver bromide and solid sodium chloride. ...
... silver chloride reacts with aqueous sodium bromide to produce aqueous silver bromide and solid sodium chloride. ...
10 TEST 2 (of 3)
... Use the ideal gas law (PV = nRT) to calculate the ideal gas law constant R at standard temperature and pressure (273 K, 1.00 atm) assuming a molar volume of 22.4 L. ...
... Use the ideal gas law (PV = nRT) to calculate the ideal gas law constant R at standard temperature and pressure (273 K, 1.00 atm) assuming a molar volume of 22.4 L. ...
Math 31 Ch. 3 Review notes
... Since the rate of reaction is the derivative of the concentration function, chemists often determine the rate of change of reaction by measuring the slope of a tangent. Rates of Change in the Social Sciences (3.4) We will look at rates of change in business and economics. Cost Function: [C(x)] = the ...
... Since the rate of reaction is the derivative of the concentration function, chemists often determine the rate of change of reaction by measuring the slope of a tangent. Rates of Change in the Social Sciences (3.4) We will look at rates of change in business and economics. Cost Function: [C(x)] = the ...
Balancing Equations
... equation has the same number of atoms of each element Is this equation balanced? Fe + O2 ...
... equation has the same number of atoms of each element Is this equation balanced? Fe + O2 ...
Chapter 11 Review sheet Name
... A chemical change in which a free element replaces and releases another element in a compound is called a(n) (10) reaction. A chemical change in which there is an exchange of ions between two compounds is called a(n) ...
... A chemical change in which a free element replaces and releases another element in a compound is called a(n) (10) reaction. A chemical change in which there is an exchange of ions between two compounds is called a(n) ...
Gizmos: Types of Reactions
... is a substance consisting of one kind of atom, such as aluminum (Al) or oxygen gas (O2). A compound is a substance made of more than one kind of atom, such as water (H2O) or table salt (NaCl). Question: How are chemical reactions classified? 1. Match: Most chemical reactions can be classified as one ...
... is a substance consisting of one kind of atom, such as aluminum (Al) or oxygen gas (O2). A compound is a substance made of more than one kind of atom, such as water (H2O) or table salt (NaCl). Question: How are chemical reactions classified? 1. Match: Most chemical reactions can be classified as one ...
Chemical Reactions and Reaction Stoichiometry
... reacting with hydrogen gas to form ammonia. How many moles of ammonia can be produced from 3 moles of nitrogen and 6 moles of hydrogen? ...
... reacting with hydrogen gas to form ammonia. How many moles of ammonia can be produced from 3 moles of nitrogen and 6 moles of hydrogen? ...
Chemistry 116: General Chemistry
... N2 has an especially strong bond between the N atoms. As a gas molecule, N2 does not often collide with other reactants. The lone pairs on N do react but only reversibly. Reactions with N2 are indeed thermodynamically favorable, but they are just slow. ...
... N2 has an especially strong bond between the N atoms. As a gas molecule, N2 does not often collide with other reactants. The lone pairs on N do react but only reversibly. Reactions with N2 are indeed thermodynamically favorable, but they are just slow. ...
Equilibrium (PowerPoint) West Coast 2015
... Students will understand and appreciate the relationship between kinetics and equilibrium. ...
... Students will understand and appreciate the relationship between kinetics and equilibrium. ...