Everything is made of atoms.
... A brief history of the atom Modern atomic theory began with the publication in 1808 by British chemist and physicist John Dalton of his experimental conclusions that: 1. all atoms of an element have the same size and weight, and 2. atoms of elements unite chemically in simple numerical ratios to fo ...
... A brief history of the atom Modern atomic theory began with the publication in 1808 by British chemist and physicist John Dalton of his experimental conclusions that: 1. all atoms of an element have the same size and weight, and 2. atoms of elements unite chemically in simple numerical ratios to fo ...
C-3 Study Guide Name PART A: Use the terms/statements from the
... 6. According to the law of definite proportions, any two samples of NaCl have 7. The principles of atomic theory recognized today were conceived by 8. According to Dalton’s atomic theory, atoms ...
... 6. According to the law of definite proportions, any two samples of NaCl have 7. The principles of atomic theory recognized today were conceived by 8. According to Dalton’s atomic theory, atoms ...
Chemistry Scavenger Hunt
... 1. All materials, whether solid, liquid or gas, are made of _______________. Atoms are the smallest ____________of ___________. Scientists have found over _____________ different kinds of atoms. The many different materials we encounter are made from _______________________ of these atoms. A materia ...
... 1. All materials, whether solid, liquid or gas, are made of _______________. Atoms are the smallest ____________of ___________. Scientists have found over _____________ different kinds of atoms. The many different materials we encounter are made from _______________________ of these atoms. A materia ...
Name Class Date Skills Worksheet Directed Reading B Section
... _____ 10. In 1911, Rutherford revised the atomic theory. Which of the following is NOT part of that theory? a. Atoms are mostly empty space. b. The nucleus is a tiny, dense, positively charged region. c. Positively charged particles that pass close by the nucleus are pushed away by the positive char ...
... _____ 10. In 1911, Rutherford revised the atomic theory. Which of the following is NOT part of that theory? a. Atoms are mostly empty space. b. The nucleus is a tiny, dense, positively charged region. c. Positively charged particles that pass close by the nucleus are pushed away by the positive char ...
Atoms ppt 8/26/16
... 4. Where are the three main subatomic particles located? 5. How do protons determine an atom’s identity? ...
... 4. Where are the three main subatomic particles located? 5. How do protons determine an atom’s identity? ...
Chapter 5
... The ATOM • Democritus (400 BC) reasoned that all things were made up of indivisible particles: ATOMS • Dalton (1766-1844) performed experiments – Atomic Theory • All elements are composed of tiny indivisible particles called ATOMS • Atoms of the same element are identical, but different from other e ...
... The ATOM • Democritus (400 BC) reasoned that all things were made up of indivisible particles: ATOMS • Dalton (1766-1844) performed experiments – Atomic Theory • All elements are composed of tiny indivisible particles called ATOMS • Atoms of the same element are identical, but different from other e ...
atom n
... Thales of Miletus discovered that a piece of amber, after rubbing it with fur, attracts bits of hair and feathers and other light objects. He suggested that this mysterious force came from the amber. Thales, however, did not connect this force with any atomic particle. Not until around 460 B.C., did ...
... Thales of Miletus discovered that a piece of amber, after rubbing it with fur, attracts bits of hair and feathers and other light objects. He suggested that this mysterious force came from the amber. Thales, however, did not connect this force with any atomic particle. Not until around 460 B.C., did ...
History of the discovery of atomic structure
... In 1920 Rutherford came up with the idea that atoms must contain a third particle. He thought this because the masses of atoms that were being measured were heavier than you would get from just the masses of protons and electrons added together. He said that this particle would have no charge and t ...
... In 1920 Rutherford came up with the idea that atoms must contain a third particle. He thought this because the masses of atoms that were being measured were heavier than you would get from just the masses of protons and electrons added together. He said that this particle would have no charge and t ...
chapter2 2012 (no naming)
... Molecules and Ions • Molecule: Two or more atoms chemically combined 1. Atoms involved are often nonmetals 2. Covalent bonds are strong forces that hold the atoms together ...
... Molecules and Ions • Molecule: Two or more atoms chemically combined 1. Atoms involved are often nonmetals 2. Covalent bonds are strong forces that hold the atoms together ...
The Size of the Atom Atomic Numbers Atomic Mass Numbers
... diagram on page 201, picturing the basic structure of the atom, is not drawn to scale. If the nucleus were as large as shown, the electron cloud would extend far beyond your classroom. The negative electrons stick around the nucleus because they are attracted to the positively charged protons. Elect ...
... diagram on page 201, picturing the basic structure of the atom, is not drawn to scale. If the nucleus were as large as shown, the electron cloud would extend far beyond your classroom. The negative electrons stick around the nucleus because they are attracted to the positively charged protons. Elect ...
The Scientists - WordPress.com
... He studied the pressure of gases, concluding that gases must consist of tiny particles in constant motion. ...
... He studied the pressure of gases, concluding that gases must consist of tiny particles in constant motion. ...
mc06sete_c03ct_018
... _____ 4. Rutherford’s gold-foil experiment led him to conclude that a. Thomson’s plum pudding model of the atom was accurate. b. alpha particles were a poor choice for a bombardment material. c. a dense region of positive charge existed somewhere in the atom. d. light was emitted by electrons return ...
... _____ 4. Rutherford’s gold-foil experiment led him to conclude that a. Thomson’s plum pudding model of the atom was accurate. b. alpha particles were a poor choice for a bombardment material. c. a dense region of positive charge existed somewhere in the atom. d. light was emitted by electrons return ...
cell molecules
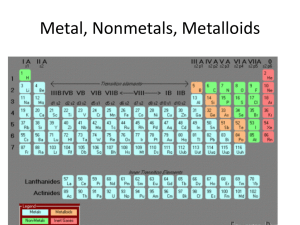
... • Each element has a unique symbol, usually from the first one or two letters of the name, often from Latin or German. ...
... • Each element has a unique symbol, usually from the first one or two letters of the name, often from Latin or German. ...
Summarised Notes
... An atom is the smallest particle of an element that has the chemical properties of that element. ...
... An atom is the smallest particle of an element that has the chemical properties of that element. ...
J.J. Thomson and the Cathode Ray Tube 1897
... single piece of gold is called an atom of gold. An atom is the smallest particle of an element that acts like the element. ...
... single piece of gold is called an atom of gold. An atom is the smallest particle of an element that acts like the element. ...
Topic 3 Review
... compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole numbers. ...
... compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole numbers. ...
CHEM 101 Dual Enrollment HW4 Question 1 of 12 Dalton`s
... Question 1 of 12 Dalton's postulates, also known as Dalton's atomic theory, include some proposals that have been updated or changed due to new discoveries. Which of the following statements were parts of Dalton's original atomic theory? Select all that apply. Atoms of the same element have the same ...
... Question 1 of 12 Dalton's postulates, also known as Dalton's atomic theory, include some proposals that have been updated or changed due to new discoveries. Which of the following statements were parts of Dalton's original atomic theory? Select all that apply. Atoms of the same element have the same ...
ATOMIC TIMELINE - Dublin Schools
... • proposed the Atomic theory of matter based on his experimental observations • His Discoveries: ...
... • proposed the Atomic theory of matter based on his experimental observations • His Discoveries: ...
PP - myndrs.com
... be made or destroyed All atoms of the same element are identical Different elements have different types of atoms Chemical reactions occur when atoms are rearranged Compounds are formed from atoms of the different elements coming together. ...
... be made or destroyed All atoms of the same element are identical Different elements have different types of atoms Chemical reactions occur when atoms are rearranged Compounds are formed from atoms of the different elements coming together. ...
Structure of an Atom structure_of_atom
... be made or destroyed All atoms of the same element are identical Different elements have different types of atoms Chemical reactions occur when atoms are rearranged Compounds are formed from atoms of the different elements coming together. ...
... be made or destroyed All atoms of the same element are identical Different elements have different types of atoms Chemical reactions occur when atoms are rearranged Compounds are formed from atoms of the different elements coming together. ...
Intro to atoms and the periodic table
... At the end of this lesson, you should be able to: Describe and define atoms and their subatomic particles Describe the relationship between atoms and elements ...
... At the end of this lesson, you should be able to: Describe and define atoms and their subatomic particles Describe the relationship between atoms and elements ...
File - J. Seguin Science
... • Who: Aristotle, Democritus • When: More than 2000 years ago • Where: Greece • What: Aristotle believed in 4 elements: Earth, Air, Fire, and Water. Democritus believed that matter was made of small particles he named “atoms”. • Why: Aristotle and Democritus used observation and inference to explain ...
... • Who: Aristotle, Democritus • When: More than 2000 years ago • Where: Greece • What: Aristotle believed in 4 elements: Earth, Air, Fire, and Water. Democritus believed that matter was made of small particles he named “atoms”. • Why: Aristotle and Democritus used observation and inference to explain ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.