pdf.format
... The yellow flash of light is due to a particles striking the screen; J.J. Thomson concluded from his Crookes Tube and CRT experiments: 1) an electron is a particle having mass 2) balance and harmony; every substance has an equal amount of positive charge for the amount of negatively charged particle ...
... The yellow flash of light is due to a particles striking the screen; J.J. Thomson concluded from his Crookes Tube and CRT experiments: 1) an electron is a particle having mass 2) balance and harmony; every substance has an equal amount of positive charge for the amount of negatively charged particle ...
Click here to Ch 3.2_ Atoms_Structure
... empty space; that atoms are indestructible, and have always been and always will be in motion; that there is an infinite number of atoms and of kinds of atoms, which differ in shape and size. Of the mass of atoms, Democritus said, "The more any indivisible exceeds, the heavier it is". ...
... empty space; that atoms are indestructible, and have always been and always will be in motion; that there is an infinite number of atoms and of kinds of atoms, which differ in shape and size. Of the mass of atoms, Democritus said, "The more any indivisible exceeds, the heavier it is". ...
JJ Thomson
... --solutions from equation yield information about probability maps or shapes on different energy levels MODEL: "wave/mechanical": basic layout of atom similar to Bohr and Rutherford, but electrons do not follow simple orbits; their position can only be predicted in terms of probability ...
... --solutions from equation yield information about probability maps or shapes on different energy levels MODEL: "wave/mechanical": basic layout of atom similar to Bohr and Rutherford, but electrons do not follow simple orbits; their position can only be predicted in terms of probability ...
2013 Final Exam Answers
... 21. As the cell operates, the cations move towards the a) the Pb electrode and the Pb electrode gains mass. b) the Pb electrode and the Pb electrode loses mass. c) the Zn electrode and the Zn electrode gains mass d) the Zn electrode and the Zn electrode loses mass e) more information i ...
... 21. As the cell operates, the cations move towards the a) the Pb electrode and the Pb electrode gains mass. b) the Pb electrode and the Pb electrode loses mass. c) the Zn electrode and the Zn electrode gains mass d) the Zn electrode and the Zn electrode loses mass e) more information i ...
KWL chart and chem notes
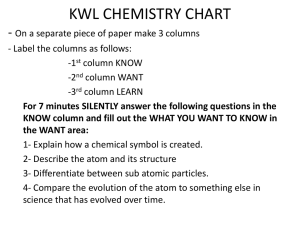
... KNOW column and fill out the WHAT YOU WANT TO KNOW in the WANT area: 1- Explain how a chemical symbol is created. 2- Describe the atom and its structure 3- Differentiate between sub atomic particles. 4- Compare the evolution of the atom to something else in science that has evolved over time. ...
... KNOW column and fill out the WHAT YOU WANT TO KNOW in the WANT area: 1- Explain how a chemical symbol is created. 2- Describe the atom and its structure 3- Differentiate between sub atomic particles. 4- Compare the evolution of the atom to something else in science that has evolved over time. ...
answers_to_questions_on_pages_100
... Answers to Questions on Pages 100-101 5. A) Protons and neutrons are found in the nucleus while the electrons orbit the nucleus in their respective energy levels. B) The protons and neutrons make up the majority of the mass of an atom. C) CORRECTION – The electrons make up most of the space as their ...
... Answers to Questions on Pages 100-101 5. A) Protons and neutrons are found in the nucleus while the electrons orbit the nucleus in their respective energy levels. B) The protons and neutrons make up the majority of the mass of an atom. C) CORRECTION – The electrons make up most of the space as their ...
• Ernest Rutherford • gold foil experiment a tiny dense postive core
... a tiny dense postive core called the nucleus surrounded by mostly empty space containing the rapidly moving negative electrons ...
... a tiny dense postive core called the nucleus surrounded by mostly empty space containing the rapidly moving negative electrons ...
theory1 (osergienko v1)
... A given compound always contains the same proportions (by mass) of elements regardless of the source For example water H2O must Always have 8 g oxygen Combining with 1 g Hydrogen Hydrogen Peroxide H2O2 must Have 16 g Oxygen combining with 1 g Hydrogen ...
... A given compound always contains the same proportions (by mass) of elements regardless of the source For example water H2O must Always have 8 g oxygen Combining with 1 g Hydrogen Hydrogen Peroxide H2O2 must Have 16 g Oxygen combining with 1 g Hydrogen ...
Chapter 13 Electrons in Atoms
... 1.Classical mechanics explains the motions of objects larger than atoms. The object gains or loses energy in any amount. 2.Quantum mechanics explains the motions of subatomic particles and atom as waves. These particles gain or lose energy in ...
... 1.Classical mechanics explains the motions of objects larger than atoms. The object gains or loses energy in any amount. 2.Quantum mechanics explains the motions of subatomic particles and atom as waves. These particles gain or lose energy in ...
Ch. 4 Slides
... we feel, what we see. • In this chapter, we trace the history of the atom and learn about its makeup. ...
... we feel, what we see. • In this chapter, we trace the history of the atom and learn about its makeup. ...
The Language of Chemistry
... • A pure substance has well defined physical and chemical properties. • Pure substances can be classified as elements or compounds. • Compounds can be further reduced into two or more elements. • Elements consist of only one type of atom. They cannot be decomposed or further simplified by ordinary m ...
... • A pure substance has well defined physical and chemical properties. • Pure substances can be classified as elements or compounds. • Compounds can be further reduced into two or more elements. • Elements consist of only one type of atom. They cannot be decomposed or further simplified by ordinary m ...
Atoms
... large models to explain something that is very small Models of the atom were used to explain data or facts that were gathered experimentally. So, these models are also theories ...
... large models to explain something that is very small Models of the atom were used to explain data or facts that were gathered experimentally. So, these models are also theories ...
Chapter 3 - CCRI Faculty Web
... with electrons revolving around in orbits of specific energies (called Energy levels) ...
... with electrons revolving around in orbits of specific energies (called Energy levels) ...
Chapt9
... more complex molecules can be polar or nonpolar, depending on their 3-D shape (Later) ...
... more complex molecules can be polar or nonpolar, depending on their 3-D shape (Later) ...
Atomic Concepts
... – “Matter can neither be created nor destroyed in a chemical reaction.” – Matter doesn't appear or disappear in a reaction, it only changes from one form to another. ...
... – “Matter can neither be created nor destroyed in a chemical reaction.” – Matter doesn't appear or disappear in a reaction, it only changes from one form to another. ...
BC1 Atoms Unit Standards
... Explain the arrangement of the elements on the Periodic Table, including the significant relationships among elements in a given column or row. (Determine an element’s placement on periodic table based on properties, number of protons, or electron configuration) Create visual models for atoms of dif ...
... Explain the arrangement of the elements on the Periodic Table, including the significant relationships among elements in a given column or row. (Determine an element’s placement on periodic table based on properties, number of protons, or electron configuration) Create visual models for atoms of dif ...
Course Outline
... Organic chemistry is the chemistry of compounds that contain the element of carbon. Organic chemistry affect every facet of our lives, and therefore it is important and useful to know something ...
... Organic chemistry is the chemistry of compounds that contain the element of carbon. Organic chemistry affect every facet of our lives, and therefore it is important and useful to know something ...
CH 115 Fall 2014Exam I Review Brief overview of topics/concepts to
... General model of an atom – what is it made of, how is it organized Dalton’s atomic theory – Be able to explain in your own words Exceptions Subatomic particles – what are they Atomic number Mass number Atomic weight Atomic mass Isotopes + calculating atomic weight of an element, vice v ...
... General model of an atom – what is it made of, how is it organized Dalton’s atomic theory – Be able to explain in your own words Exceptions Subatomic particles – what are they Atomic number Mass number Atomic weight Atomic mass Isotopes + calculating atomic weight of an element, vice v ...
Ch 2 notes
... • Atoms of an element are not changed into atoms of a different element by chemical reactions; atoms are neither created nor destroyed in chemical reactions. ...
... • Atoms of an element are not changed into atoms of a different element by chemical reactions; atoms are neither created nor destroyed in chemical reactions. ...
ATOM ATOMIC SYMBOL ATOMIC NUMBER
... Number of Protons = Atomic Number (Use the large colored marshmallows for protons) Number of Neutrons = Atomic Mass – Atomic Number (Use the large white marshmallows for neutrons) Number of Electrons = Number of Protons (Use the small colored marshmallows for electrons) ...
... Number of Protons = Atomic Number (Use the large colored marshmallows for protons) Number of Neutrons = Atomic Mass – Atomic Number (Use the large white marshmallows for neutrons) Number of Electrons = Number of Protons (Use the small colored marshmallows for electrons) ...
Chem vocab quiz definitons
... Metal is the most common classification of elements. A group or family is a combination of elements that are shown as a column in the Periodic Table of Elements. These elements share the same number of valence electrons and react with the same elements. Metalloids are the elements located along the ...
... Metal is the most common classification of elements. A group or family is a combination of elements that are shown as a column in the Periodic Table of Elements. These elements share the same number of valence electrons and react with the same elements. Metalloids are the elements located along the ...
Evolution of Atomic Theory
... billiard ball a small, solid sphere. Thomson’s work suggested that it wasn’t one particle but a “jigsaw puzzle” of smaller pieces. Thomson discovered the electron by using a cathode-ray tube, He interpreted that the deflection of the rays by electrically charged plates and magnets as evidence of "bo ...
... billiard ball a small, solid sphere. Thomson’s work suggested that it wasn’t one particle but a “jigsaw puzzle” of smaller pieces. Thomson discovered the electron by using a cathode-ray tube, He interpreted that the deflection of the rays by electrically charged plates and magnets as evidence of "bo ...
The periodic table is the most significant tool that chemist use for
... vigorously and stimulating much new work in chemistry. His insistence that elements with similar characteristics be listed in the same families forced him to leave several blank spaces in his table. Mendeleev boldly predicted their existence and properties. ...
... vigorously and stimulating much new work in chemistry. His insistence that elements with similar characteristics be listed in the same families forced him to leave several blank spaces in his table. Mendeleev boldly predicted their existence and properties. ...
here
... After reading that section, click the link for Section 2d, “More Electron Basics” and read through this page. Pay attention to how ionization energy, electronegativity and atomic size (radius) are impacted as you move along the periodic table. ...
... After reading that section, click the link for Section 2d, “More Electron Basics” and read through this page. Pay attention to how ionization energy, electronegativity and atomic size (radius) are impacted as you move along the periodic table. ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.