Pathways of Amino Acid Degredation
... If it be, indeed, the case that in alkaptonuria and the other conditions mentioned we are dealing with individualities of metabolism and not with the results of morbid processes the thought naturally presents itself that these are merely extreme examples of variations of chemical behaviour which are ...
... If it be, indeed, the case that in alkaptonuria and the other conditions mentioned we are dealing with individualities of metabolism and not with the results of morbid processes the thought naturally presents itself that these are merely extreme examples of variations of chemical behaviour which are ...
Document
... If it be, indeed, the case that in alkaptonuria and the other conditions mentioned we are dealing with individualities of metabolism and not with the results of morbid processes the thought naturally presents itself that these are merely extreme examples of variations of chemical behaviour which are ...
... If it be, indeed, the case that in alkaptonuria and the other conditions mentioned we are dealing with individualities of metabolism and not with the results of morbid processes the thought naturally presents itself that these are merely extreme examples of variations of chemical behaviour which are ...
Biochemistry WS.1
... b) How would you test for glucose? ______________________________________________________________ c) How would you test for starch? ______________________________________________________________ ...
... b) How would you test for glucose? ______________________________________________________________ c) How would you test for starch? ______________________________________________________________ ...
Today: Membrane Structure continued Membrane Transport Exam
... intermediates. Phosphorylation usually makes a molecule less stable/more reactive. ...
... intermediates. Phosphorylation usually makes a molecule less stable/more reactive. ...
Biology STAAR EOC Review Sheets Alief
... them to the skeletal system to control the amount of calcium released from bones. Food is broken down in the stomach mechanically by the muscular system (churns food) and chemically by water, acid, and enzymes in the digestive system; nutrients are then absorbed by blood in the circulatory system Ce ...
... them to the skeletal system to control the amount of calcium released from bones. Food is broken down in the stomach mechanically by the muscular system (churns food) and chemically by water, acid, and enzymes in the digestive system; nutrients are then absorbed by blood in the circulatory system Ce ...
Chemical Reactions and Enzymes
... Two paths will take you to the same end location. Which one will take less energy to get there and will happen faster? ...
... Two paths will take you to the same end location. Which one will take less energy to get there and will happen faster? ...
6.1 Digestion and absorption assessment statements
... Pancreatic endopeptidase – proteolytic enzymes that hydrolyze internal peptide bonds digesting proteins/polypeptides into shorter amino acid chains. Trypsin that works at pH 8 is an example. Explain the need for enzymes to digest most macromolecules in food into monomers in the small intestine. ...
... Pancreatic endopeptidase – proteolytic enzymes that hydrolyze internal peptide bonds digesting proteins/polypeptides into shorter amino acid chains. Trypsin that works at pH 8 is an example. Explain the need for enzymes to digest most macromolecules in food into monomers in the small intestine. ...
chapter 19
... pI? Be able to draw an amino acid in the proper form at a particular pH, if given its pI. 5. How can electrophoresis be used to separate mixtures of amino acids? 6. Define C terminal, N terminal, peptide, peptide bond. 7. Define primary, secondary, tertiary, and quaternary structure. Define alpha he ...
... pI? Be able to draw an amino acid in the proper form at a particular pH, if given its pI. 5. How can electrophoresis be used to separate mixtures of amino acids? 6. Define C terminal, N terminal, peptide, peptide bond. 7. Define primary, secondary, tertiary, and quaternary structure. Define alpha he ...
CHAPTER-II ENZYMES
... This may result in different enzymes, called isozymes, with the same function having the same basic name. Isoenzymes have a different amino acid sequence and might be distinguished by their optimal pH, kinetic properties or immunologically. Isoenzyme and isozyme are homologous proteins. Furthermore, ...
... This may result in different enzymes, called isozymes, with the same function having the same basic name. Isoenzymes have a different amino acid sequence and might be distinguished by their optimal pH, kinetic properties or immunologically. Isoenzyme and isozyme are homologous proteins. Furthermore, ...
Enzymes & pH - SchoolWorld an Edline Solution
... Enzyme shape allows only certain reactants (substrates) to bind to the enzyme – Substrate: the specific reactants that an enzyme acts on – Active site: the part of the enzyme where the substrate connects. Specific to only one substrate! • Substrate + active site = like a lock and key! ...
... Enzyme shape allows only certain reactants (substrates) to bind to the enzyme – Substrate: the specific reactants that an enzyme acts on – Active site: the part of the enzyme where the substrate connects. Specific to only one substrate! • Substrate + active site = like a lock and key! ...
key
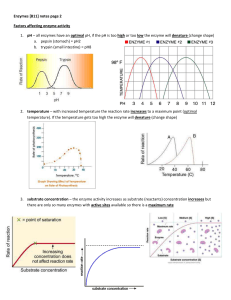
... 1. pH – all enzymes have an optimal pH, if the pH is too high or too low the enzyme will denature (change shape) a. pepsin (stomach) = pH2 ...
... 1. pH – all enzymes have an optimal pH, if the pH is too high or too low the enzyme will denature (change shape) a. pepsin (stomach) = pH2 ...
Proteins
... 1. Enzyme forms a temporary association with the substrate or substrates 2. The Enzyme and Substrate form a close physical association between the molecules called an enzyme substrate complex. ...
... 1. Enzyme forms a temporary association with the substrate or substrates 2. The Enzyme and Substrate form a close physical association between the molecules called an enzyme substrate complex. ...
What are the strain properties (C3025)? | NEB
... FAQ: What are the strain properties (C3025)? The properties of this strain that contribute to its usefulness as a protein expression strain are described below. The genotypes underlying these properties appear in parentheses. Lac Promoter Control (lacIq): The lac repressor blocks expression from lac ...
... FAQ: What are the strain properties (C3025)? The properties of this strain that contribute to its usefulness as a protein expression strain are described below. The genotypes underlying these properties appear in parentheses. Lac Promoter Control (lacIq): The lac repressor blocks expression from lac ...
B. Enzymes have four features
... in “ase” B. Enzymes have four features: 1. Enzymes speed up reactions. 2. Enzymes can be reused. 3. Enzymes, at least some of them, can recognize both reactants and products in order to catalyze a reaction in both directions. 4. Enzymes are very selective about the substrates to which they will bind ...
... in “ase” B. Enzymes have four features: 1. Enzymes speed up reactions. 2. Enzymes can be reused. 3. Enzymes, at least some of them, can recognize both reactants and products in order to catalyze a reaction in both directions. 4. Enzymes are very selective about the substrates to which they will bind ...
Enzymes
... • Describe how changing temperature and pH will change the rate of reaction of an enzyme-catalysed reaction (C) • Explain the specificity of enzymes in terms of the ‘lock and key’ mechanism (B) • Explain how enzyme activity is affected by pH and temperature (A) • Link the effect of temperature and p ...
... • Describe how changing temperature and pH will change the rate of reaction of an enzyme-catalysed reaction (C) • Explain the specificity of enzymes in terms of the ‘lock and key’ mechanism (B) • Explain how enzyme activity is affected by pH and temperature (A) • Link the effect of temperature and p ...
Document
... (NH2) with an oxygen (O) atom N-, O-, or S-Dealkylation – replacement of an alkyl group (e.g., CH3) with a hydrogen atom. Typically, the alkyl group in the parent molecule is bonded to a N, O, or S atom. Aliphatic or aromatic hydroxylation – addition of a hydroxyl group (OH) to a molecule ...
... (NH2) with an oxygen (O) atom N-, O-, or S-Dealkylation – replacement of an alkyl group (e.g., CH3) with a hydrogen atom. Typically, the alkyl group in the parent molecule is bonded to a N, O, or S atom. Aliphatic or aromatic hydroxylation – addition of a hydroxyl group (OH) to a molecule ...
3.2.1 enzymes - Haiku Learning : Login
... • Release of the products restores the enzyme to its original form. • The enzyme can repeat this reaction over and over, as long as substrate molecules are present. ...
... • Release of the products restores the enzyme to its original form. • The enzyme can repeat this reaction over and over, as long as substrate molecules are present. ...
What are enzymes?
... enzymes are added to an industrial process. If you are using chemicals as a catalyst, you have to put up with a bundle of side effects because chemicals are non-specific. The chemicals will do their thing to whatever they come across. When an enzyme does the job, there are no side effects. For insta ...
... enzymes are added to an industrial process. If you are using chemicals as a catalyst, you have to put up with a bundle of side effects because chemicals are non-specific. The chemicals will do their thing to whatever they come across. When an enzyme does the job, there are no side effects. For insta ...
ENZYMES MAKE THE WORLD GO `ROUND
... CAN YOU STOP THEM? Good question! We know what you're thinking. What if enzymes just kept going and converted every molecule in the world? They would never stop... like a monster! There are many factors that can regulate enzyme activity, including temperature, pH levels, and inhibitors. ...
... CAN YOU STOP THEM? Good question! We know what you're thinking. What if enzymes just kept going and converted every molecule in the world? They would never stop... like a monster! There are many factors that can regulate enzyme activity, including temperature, pH levels, and inhibitors. ...
Review for Practical (Solutions) Enzyme A will outcompete Enzyme
... (98 g)/(1.87 g/ml) = 52.4 ml. So, I’d add 52.4 ml to water and bring the volume up to 1 L. Percentages: 98g/1000 ml = 9.8g/100 ml = 9.8%(w/v); 52.4 ml/1000 ml = 5.24 ml/100 ml = 5.24% (v/v) Normality: 1 M solution = 3N ...
... (98 g)/(1.87 g/ml) = 52.4 ml. So, I’d add 52.4 ml to water and bring the volume up to 1 L. Percentages: 98g/1000 ml = 9.8g/100 ml = 9.8%(w/v); 52.4 ml/1000 ml = 5.24 ml/100 ml = 5.24% (v/v) Normality: 1 M solution = 3N ...
Enzymes: The Biological Catalysts
... D. When an enzyme binds with the substrate, the bonded substrate interacts with the enzyme causing it to change shape. This change in shape facilitates the chemical reaction to occur. This is called the induced fit. ...
... D. When an enzyme binds with the substrate, the bonded substrate interacts with the enzyme causing it to change shape. This change in shape facilitates the chemical reaction to occur. This is called the induced fit. ...
Beta-lactamase

Beta-lactamases are enzymes (EC 3.5.2.6) produced by some bacteria that provide resistance to β-lactam antibiotics like penicillins, cephamycins, and carbapenems (ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a β-lactam. Through hydrolysis, the lactamase enzyme breaks the β-lactam ring open, deactivating the molecule's antibacterial properties.Beta-lactam antibiotics are typically used to treat a broad spectrum of Gram-positive and Gram-negative bacteria.Beta-lactamases produced by Gram-negative organisms are usually secreted, especially when antibiotics are present in the environment.