Physics 102, Class 11 “The Atomic Nature of Matter” Physics 102
... – A: found that negative electricity was transported by tiny particles – B: found the first particles smaller than the hydrogen atom – C: found that negative electricity particles were always exactly the same, regardless of the materials used to make the electrodes or the gas in the tube review the ...
... – A: found that negative electricity was transported by tiny particles – B: found the first particles smaller than the hydrogen atom – C: found that negative electricity particles were always exactly the same, regardless of the materials used to make the electrodes or the gas in the tube review the ...
CH 2 development of atomic theory
... The compete symbol for an atom or ion consist of the elemental symbol surrounded by subscripts and superscripts. The subscripts are Z and y; the super scripts are A and w. A and Z are used for describing information about atomic species. W is used for ions, and y is used for writing formulas of comp ...
... The compete symbol for an atom or ion consist of the elemental symbol surrounded by subscripts and superscripts. The subscripts are Z and y; the super scripts are A and w. A and Z are used for describing information about atomic species. W is used for ions, and y is used for writing formulas of comp ...
Historical Introduction to the Elementary Particles 2
... For example, p denotes the proton and p the antiproton; n the neutron and n the antineutron. However, in some cases it is more customary simply to specify the charge. Thus most people write e+ for the positron (not e) and m+ for the antimuon (not m). [But you must not mix conventions: e + is ambiguo ...
... For example, p denotes the proton and p the antiproton; n the neutron and n the antineutron. However, in some cases it is more customary simply to specify the charge. Thus most people write e+ for the positron (not e) and m+ for the antimuon (not m). [But you must not mix conventions: e + is ambiguo ...
Primary electrons make random elastic and inelastic collision either
... decreases the kinetic energy of the bombarding electron; meanwhile the deviation of its path is very small… ...
... decreases the kinetic energy of the bombarding electron; meanwhile the deviation of its path is very small… ...
Bohr`s model of the atom
... back and forth off the mirrors. As they pass through the crystal, they stimulate emission in other atoms. ...
... back and forth off the mirrors. As they pass through the crystal, they stimulate emission in other atoms. ...
Bohr`s Model of the Atom
... concentration of charge could produce the electric field strong enough to cause the heavy deflection of alpha particles observed. ...
... concentration of charge could produce the electric field strong enough to cause the heavy deflection of alpha particles observed. ...
Superconcepts
... x. Schrödinger showed that atomic wave spectra (electrons) could be predicted by wave functions in 1926. xi. Heisinger found that atomic properties were ‘indeterminant’ or uncertain in 1927. xii. When the double-slit experiment was repeated with electrons, the results confirmed that electrons behave ...
... x. Schrödinger showed that atomic wave spectra (electrons) could be predicted by wave functions in 1926. xi. Heisinger found that atomic properties were ‘indeterminant’ or uncertain in 1927. xii. When the double-slit experiment was repeated with electrons, the results confirmed that electrons behave ...
document
... Part A: Match the letter of the correct definition to the Vocabulary term. 1. Octet Rule A. A reaction in which one substance breaks down into its parts. 2. Ion B. A bond that is formed by sharing electrons. 3. Charge C. A charged atom. D. The number of electrons an 4. Covalent Bond element is willi ...
... Part A: Match the letter of the correct definition to the Vocabulary term. 1. Octet Rule A. A reaction in which one substance breaks down into its parts. 2. Ion B. A bond that is formed by sharing electrons. 3. Charge C. A charged atom. D. The number of electrons an 4. Covalent Bond element is willi ...
The study of biology can help you better understand
... Answer question in the textbook: Ch 5 RQ 47; 50; 56;58;61;80; 102, 103,104 ...
... Answer question in the textbook: Ch 5 RQ 47; 50; 56;58;61;80; 102, 103,104 ...
Unit 1 - Measurement Atomic Theory
... (ii) Color comes from visible light NOT absorbed. (d) OTHER PROPERTIES: (i) Boiling Point, Melting Point, Malleability, Ductility, Specific Gravity, luster, vapor pressure, etc. ...
... (ii) Color comes from visible light NOT absorbed. (d) OTHER PROPERTIES: (i) Boiling Point, Melting Point, Malleability, Ductility, Specific Gravity, luster, vapor pressure, etc. ...
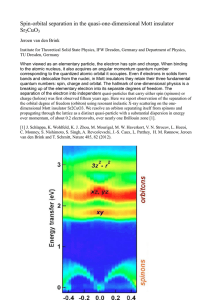
Spin-orbital separation in the quasi-one
... When viewed as an elementary particle, the electron has spin and charge. When binding to the atomic nucleus, it also acquires an angular momentum quantum number corresponding to the quantized atomic orbital it occupies. Even if electrons in solids form bands and delocalize from the nuclei, in Mott i ...
... When viewed as an elementary particle, the electron has spin and charge. When binding to the atomic nucleus, it also acquires an angular momentum quantum number corresponding to the quantized atomic orbital it occupies. Even if electrons in solids form bands and delocalize from the nuclei, in Mott i ...
551Lect03
... The outer electrons are weakly bound. They roam freely in the space between the atoms and thus are able to conduct electricity. They can be approximated by free electrons in a constant, attractive “inner potential” V0 (typically -15 eV). ...
... The outer electrons are weakly bound. They roam freely in the space between the atoms and thus are able to conduct electricity. They can be approximated by free electrons in a constant, attractive “inner potential” V0 (typically -15 eV). ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.