Quanta: a new view of the world
... adequately differentiated from the related quantities of force and motion. It was generally agreed that some agent of motion and change must exist; Descartes suggested, for example, that God, when creating the world, had filled it with "vortices" whose motions never ceased, but which could be transf ...
... adequately differentiated from the related quantities of force and motion. It was generally agreed that some agent of motion and change must exist; Descartes suggested, for example, that God, when creating the world, had filled it with "vortices" whose motions never ceased, but which could be transf ...
Spectral Lines - Transcript
... might be useful corollary information. There may be occasional figures that suggest what might be on the screen at that time. ...
... might be useful corollary information. There may be occasional figures that suggest what might be on the screen at that time. ...
Camp 1 - Quynh Nguyen Official Website
... Separation of the components of a mixture by physical means by using a porous medium, such as filter paper, to separate components based upon relative particles sizes. Filtration is based on the physical properties of a mixture: The particle sizes of a component to be separated must be significantly ...
... Separation of the components of a mixture by physical means by using a porous medium, such as filter paper, to separate components based upon relative particles sizes. Filtration is based on the physical properties of a mixture: The particle sizes of a component to be separated must be significantly ...
34.) Write out the set of four quantum numbers for the last electron
... 11.) Potassium iodide completely dissolved in water 12.) Soil 13.) Chromium * Classify as chemical or physical changes. 14.) Shredding cheese 15.) Melting cheese 16.) Digesting cheese 17.) Making salt from sodium and chlorine 18.) Sprinkling salt on french fries * In what group (give number) are eac ...
... 11.) Potassium iodide completely dissolved in water 12.) Soil 13.) Chromium * Classify as chemical or physical changes. 14.) Shredding cheese 15.) Melting cheese 16.) Digesting cheese 17.) Making salt from sodium and chlorine 18.) Sprinkling salt on french fries * In what group (give number) are eac ...
key
... Placed in table above using blue electrons. We predict it to be a colorless gas with low electrical conductivity and high electrical reactivity. c) Are there any elements that have not yet been discovered? If so, what would their properties be? This table has room for four more elements. The element ...
... Placed in table above using blue electrons. We predict it to be a colorless gas with low electrical conductivity and high electrical reactivity. c) Are there any elements that have not yet been discovered? If so, what would their properties be? This table has room for four more elements. The element ...
Masterton and Hurley Chapter 3
... The compound that gives vinegar its sour taste is acetic acid, which contains the elements carbon, hydrogen, and oxygen. When 5.00g of acetic acid is analyzed it is found to contain 2.00g of carbon, 0.336g of hydrogen, and 2.66g of oxygen. What is the empirical formula of acetic acid? ...
... The compound that gives vinegar its sour taste is acetic acid, which contains the elements carbon, hydrogen, and oxygen. When 5.00g of acetic acid is analyzed it is found to contain 2.00g of carbon, 0.336g of hydrogen, and 2.66g of oxygen. What is the empirical formula of acetic acid? ...
is the “quantum number”
... quantum numbers also result in small energy differences • Pauli exclusion principle: no two electrons in the same atom can be in the same quantum state • Electrons are grouped into shells and subshells • Periodic table reflects shell structure Atoms with the same number of electrons in their outer s ...
... quantum numbers also result in small energy differences • Pauli exclusion principle: no two electrons in the same atom can be in the same quantum state • Electrons are grouped into shells and subshells • Periodic table reflects shell structure Atoms with the same number of electrons in their outer s ...
Контрольная работа для 2 курса заочного отделения (физич
... In physics and chemistry, wave–particle duality is the concept that all matter and energy exhibits both wave-like and particle-like properties. A central concept of quantum mechanics, duality addresses the inadequacy of classical concepts like "particle" and "wave" in fully describing the behaviour ...
... In physics and chemistry, wave–particle duality is the concept that all matter and energy exhibits both wave-like and particle-like properties. A central concept of quantum mechanics, duality addresses the inadequacy of classical concepts like "particle" and "wave" in fully describing the behaviour ...
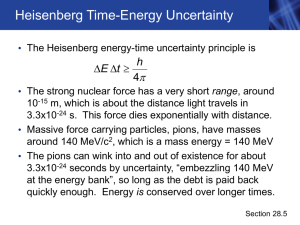
Chapter 28 - Purdue Physics
... molecule can absorb a photon only if the photon energy precisely matches the pigment energy level • More realistically (C), a range of energies is absorbed • Quantum mechanics and the existence of quantized energies for both photons and pigment molecules are ...
... molecule can absorb a photon only if the photon energy precisely matches the pigment energy level • More realistically (C), a range of energies is absorbed • Quantum mechanics and the existence of quantized energies for both photons and pigment molecules are ...
2 - FacultyWeb
... • Sharing of electrons may be equal or unequal • Atoms with six or seven valence shell electrons are electronegative, e.g., oxygen • Atoms with one or two valence shell electrons are electropositive, e.g., sodium Equal sharing of electrons produces electrically balanced nonpolar ...
... • Sharing of electrons may be equal or unequal • Atoms with six or seven valence shell electrons are electronegative, e.g., oxygen • Atoms with one or two valence shell electrons are electropositive, e.g., sodium Equal sharing of electrons produces electrically balanced nonpolar ...
transparencies - Indico
... Astronomers don't know precisely where all these particles come from. The sun is a known source of cosmic rays which are produced by high energy explosions (solar flares) in the sun's atmosphere, but the amount of cosmic rays emitted by the sun is too low to account for all the cosmic rays which str ...
... Astronomers don't know precisely where all these particles come from. The sun is a known source of cosmic rays which are produced by high energy explosions (solar flares) in the sun's atmosphere, but the amount of cosmic rays emitted by the sun is too low to account for all the cosmic rays which str ...
Physics 120 Homework Set #1 (due Sunday
... The double-slit experiment is an example of light behaving as a wave. If light was just a stream of particles we would expect a simple shadow of the two slits to appear in the screen. However, if the slit separation is comparable to the wavelength of the electromagnetic radiation, an interference pa ...
... The double-slit experiment is an example of light behaving as a wave. If light was just a stream of particles we would expect a simple shadow of the two slits to appear in the screen. However, if the slit separation is comparable to the wavelength of the electromagnetic radiation, an interference pa ...
Quantum Mechanics
... The Bohr Model of the Atom Neils Bohr described an atom with quantized energy levels. These are discrete energy levels. Since we cannot tell both location and momentum of an electron at the same time (Heisenberg Principle), this model serves to predict the probabilities of where the electrons in an ...
... The Bohr Model of the Atom Neils Bohr described an atom with quantized energy levels. These are discrete energy levels. Since we cannot tell both location and momentum of an electron at the same time (Heisenberg Principle), this model serves to predict the probabilities of where the electrons in an ...
Physics Week 15(Sem. 2)
... way the earth spins around the sun, the electrons spin around the nucleus. Therefore ...
... way the earth spins around the sun, the electrons spin around the nucleus. Therefore ...
Chapter 6: Electrostatics End of Chapter Questions
... That when something is charged, no electrons are created or destroyed, but are simply transferred from one material to another. That there is a smallest unit of charge, wherein all charged objects are some whole-number multiple of this smallest unit. The electron. ...
... That when something is charged, no electrons are created or destroyed, but are simply transferred from one material to another. That there is a smallest unit of charge, wherein all charged objects are some whole-number multiple of this smallest unit. The electron. ...
Figure 2: Alternative Periodic Table
... Placed in table above using blue electrons. We predict it to be a colorless gas with low electrical conductivity and high electrical reactivity. c) Are there any elements that have not yet been discovered? If so, what would their properties be? This table has room for four more elements. The element ...
... Placed in table above using blue electrons. We predict it to be a colorless gas with low electrical conductivity and high electrical reactivity. c) Are there any elements that have not yet been discovered? If so, what would their properties be? This table has room for four more elements. The element ...
Lecture 5. Radiation and energy. 1. The most important aspects of
... Chemistry is the study of atoms-how these basic units combine, and how substances made of atoms are changed into other substances. According to atomic theory, all matter, whether solid, liquid, or gas, is composed of particles called atoms. The simplest view of the atom is that it consists of a tiny ...
... Chemistry is the study of atoms-how these basic units combine, and how substances made of atoms are changed into other substances. According to atomic theory, all matter, whether solid, liquid, or gas, is composed of particles called atoms. The simplest view of the atom is that it consists of a tiny ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI M.Sc. THIRD
... 16. Give a detailed account of the fundamental postulates of Quantum Mechanics. 17. Using commutator algebra, obtain Heisenberg’s uncertainty relation. 18. Using the theory of particle in a potential well, well, show that a quantum particle has finite probability to exist in ...
... 16. Give a detailed account of the fundamental postulates of Quantum Mechanics. 17. Using commutator algebra, obtain Heisenberg’s uncertainty relation. 18. Using the theory of particle in a potential well, well, show that a quantum particle has finite probability to exist in ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.