view pdf - Sub-Structure of the Electron
... Several approaches for a quantum reality of the electron have been published, e.g. a model of Paul Dirac1 with mass less particles circulating each other with the speed of light or models which regard the electron as a solid charged shell rotating with the speed of light2. Diracs approach recently ...
... Several approaches for a quantum reality of the electron have been published, e.g. a model of Paul Dirac1 with mass less particles circulating each other with the speed of light or models which regard the electron as a solid charged shell rotating with the speed of light2. Diracs approach recently ...
Document
... The following pages introduce the background to the Standard Model of particle physics. It is important to realise that our current model of physics is constantly changing and being updated and improved as new experimental evidence is found. What is the world made of? The ancient Greeks believed the ...
... The following pages introduce the background to the Standard Model of particle physics. It is important to realise that our current model of physics is constantly changing and being updated and improved as new experimental evidence is found. What is the world made of? The ancient Greeks believed the ...
Observation of the Pairing Gap in a Strongly Interacting Quantum... Fermionic Atoms
... James Franck Institute, Department of Physics, University of Chicago, Chicago, IL 60637, USA Institut für Experimentalphysik, Universität Innsbruck, A-6020 Innsbruck, Austria ...
... James Franck Institute, Department of Physics, University of Chicago, Chicago, IL 60637, USA Institut für Experimentalphysik, Universität Innsbruck, A-6020 Innsbruck, Austria ...
Chapter 2 Atomic structure and spectra
... These considerations can easily be generalized to situations with more than two unpaired electrons. In atoms with configurations with three unpaired electrons, such as, for instance, N ((1s)2 (2s)2 (2p)3 ), quartet (S = 3/2) and doublet (S = 1/2) states result. ...
... These considerations can easily be generalized to situations with more than two unpaired electrons. In atoms with configurations with three unpaired electrons, such as, for instance, N ((1s)2 (2s)2 (2p)3 ), quartet (S = 3/2) and doublet (S = 1/2) states result. ...
Mole Concept and Stoichiometry
... reality, Avogadro built a theoretical foundation for determining accurate atomic and molecular masses. The concept of mole did not even exist in Avogadro’s time. Much of Avogadro’s work was based on that of Gay-Lussac ( 1778 – 1850 ) . Gay – Lussac developed the law of combining volumes which states ...
... reality, Avogadro built a theoretical foundation for determining accurate atomic and molecular masses. The concept of mole did not even exist in Avogadro’s time. Much of Avogadro’s work was based on that of Gay-Lussac ( 1778 – 1850 ) . Gay – Lussac developed the law of combining volumes which states ...
Animation of figure 40.1: Exciton formation
... in the valence band of an intrinsic semiconductor, due to the absorption of a photon of appropriate energy. The electron-hole pair exert attractive forces on each other and behave like a combined entity, called the Exciton, which has some similarities to the hydrogen atom in view of the number of pa ...
... in the valence band of an intrinsic semiconductor, due to the absorption of a photon of appropriate energy. The electron-hole pair exert attractive forces on each other and behave like a combined entity, called the Exciton, which has some similarities to the hydrogen atom in view of the number of pa ...
Chapter 3 Chemical Compounds
... i) An empirical formula is the simplest formula for a compound; it shows the types of atoms present and their relative numbers. Compounds with different molecular formulas can have the same empirical formulas and such substances will have the same percentage composition. E.g. Acetic acid (C2H4O2), f ...
... i) An empirical formula is the simplest formula for a compound; it shows the types of atoms present and their relative numbers. Compounds with different molecular formulas can have the same empirical formulas and such substances will have the same percentage composition. E.g. Acetic acid (C2H4O2), f ...
Quantum Manipulation of Ultracold Atoms—V. Vuletic
... single atom. Here F is the finesse of the resonator, w the waist size of the TEM00 resonator mode, k the wavenumber of the emitted light, and ∆Ω/4π = 2/(k2w2) the fractional solid angle subtended by the cavity mode. The success probability for emission of the read photon into the resonator, arising ...
... single atom. Here F is the finesse of the resonator, w the waist size of the TEM00 resonator mode, k the wavenumber of the emitted light, and ∆Ω/4π = 2/(k2w2) the fractional solid angle subtended by the cavity mode. The success probability for emission of the read photon into the resonator, arising ...
Chapter 18 Review 18.1 Oxidation-Reduction Reactions Oxidation
... Corrosion- process of returning metals to their natural state Cathodic Protection- the connection of an active metal to another to prevent corrosion - corrosion involves the oxidation of metals - this process creates great economic lose - most metals produce a thin oxide coating, which protect their ...
... Corrosion- process of returning metals to their natural state Cathodic Protection- the connection of an active metal to another to prevent corrosion - corrosion involves the oxidation of metals - this process creates great economic lose - most metals produce a thin oxide coating, which protect their ...
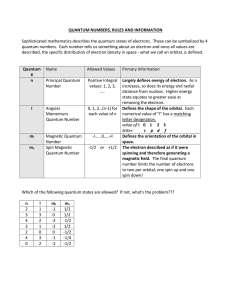
3.4 Quantum Numbers
... The Magnetic Quantum Number (ml) • Gives the exact orbital • Describes the orientation of an atomic orbital in space (how it lines up on the xyz plane) • ml can have integer values from –l to +l including 0 • The Zeemen effect showed that if a gas discharge tube was placed near a strong magnet some ...
... The Magnetic Quantum Number (ml) • Gives the exact orbital • Describes the orientation of an atomic orbital in space (how it lines up on the xyz plane) • ml can have integer values from –l to +l including 0 • The Zeemen effect showed that if a gas discharge tube was placed near a strong magnet some ...
Chemical Reactions
... Synthesis Reaction • Synthesis – 2 substances (reactants) combine to form a new substance (product). – Substances are either atoms (elements) or compounds in this case. A + ...
... Synthesis Reaction • Synthesis – 2 substances (reactants) combine to form a new substance (product). – Substances are either atoms (elements) or compounds in this case. A + ...
2.8 M - Thierry Karsenti
... technological applications thereof. For example, the fact that each element has its own characteristic “fingerprint” spectrum has contributed significantly to advances in material science and also in cosmology. ...
... technological applications thereof. For example, the fact that each element has its own characteristic “fingerprint” spectrum has contributed significantly to advances in material science and also in cosmology. ...
Atoms, Elements and Compounds Home
... All students are expected to complete the bronze level tasks. These are designed to consolidate students’ knowledge of the key concepts met in the unit. Students who complete the all the bronze level tasks to a suitable standard will be rewarded with one house point. We would expect most students to ...
... All students are expected to complete the bronze level tasks. These are designed to consolidate students’ knowledge of the key concepts met in the unit. Students who complete the all the bronze level tasks to a suitable standard will be rewarded with one house point. We would expect most students to ...
Atomic Physics
... technological applications thereof. For example, the fact that each element has its own characteristic “fingerprint” spectrum has contributed significantly to advances in material science and also in cosmology. ...
... technological applications thereof. For example, the fact that each element has its own characteristic “fingerprint” spectrum has contributed significantly to advances in material science and also in cosmology. ...
pptx - Yale University
... techniques are not quite good enough yet to study how electrons are distributed in bonds. Because light is scattered predominantly by the charged particles with the smallest mass, the electron distribution in molecules can be determined by x-ray diffraction. The roles of molecular pattern and crysta ...
... techniques are not quite good enough yet to study how electrons are distributed in bonds. Because light is scattered predominantly by the charged particles with the smallest mass, the electron distribution in molecules can be determined by x-ray diffraction. The roles of molecular pattern and crysta ...
Introduction - CNC Science
... Example of an Empirical formula from analytical data On analysis a compound is found to contain 68.85% carbon ; 4.92% hydrogen ; 26.23% Oxygen What is the empirical formula ? Answer: Imagine we have 100g of the compound then the ratios in grams is ...
... Example of an Empirical formula from analytical data On analysis a compound is found to contain 68.85% carbon ; 4.92% hydrogen ; 26.23% Oxygen What is the empirical formula ? Answer: Imagine we have 100g of the compound then the ratios in grams is ...
The Living Planet
... proton’s positive charge. Sometimes, the oxygen pulls the sheet completely away from the hydrogen; now they aren’t sharing the electron-pair at all; the hydrogen nucleus is ‘naked’ – just 1 proton – existing as an independent H+ ion. The oxygen (and the other hydrogen) now have an extra electron and ...
... proton’s positive charge. Sometimes, the oxygen pulls the sheet completely away from the hydrogen; now they aren’t sharing the electron-pair at all; the hydrogen nucleus is ‘naked’ – just 1 proton – existing as an independent H+ ion. The oxygen (and the other hydrogen) now have an extra electron and ...
Chapter 7. Dynamics of Systems of Particles
... At that time, the mass mb of the first-stage fuel container is released into space, and the second-stage rocket ignites. We now have ma for the starting mass of space ship (second stage), and m2 for its mass after the second-stage fuel container has burnt out. The terminal velocity is given by !m $ ...
... At that time, the mass mb of the first-stage fuel container is released into space, and the second-stage rocket ignites. We now have ma for the starting mass of space ship (second stage), and m2 for its mass after the second-stage fuel container has burnt out. The terminal velocity is given by !m $ ...
Practice Final Exam
... b) He was uncertain as to the applicability of quantum mechanics in understanding atoms. c) He was uncertain as to whether particles could have wave-like characteristics. d) He wasn’t uncertain. He just said that if a photon collides with an electron at rest, some of the photon’s energy will be tran ...
... b) He was uncertain as to the applicability of quantum mechanics in understanding atoms. c) He was uncertain as to whether particles could have wave-like characteristics. d) He wasn’t uncertain. He just said that if a photon collides with an electron at rest, some of the photon’s energy will be tran ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.