File
... grams. (HINT: If not given grams, use 100 grams of compound to start.) Convert the composition in grams to moles by using molar masses of the elements. (This gives a mole ratio.) Use the numbers from mole ratio to get the smallest possible whole number ...
... grams. (HINT: If not given grams, use 100 grams of compound to start.) Convert the composition in grams to moles by using molar masses of the elements. (This gives a mole ratio.) Use the numbers from mole ratio to get the smallest possible whole number ...
Hints for Names and Formulas (Ch. 4 in Zumdahl Chemistry)
... (1) ionic compounds are never called molecules and have covalent bonds only in their polyatomic ions (2) ionic compounds are generally classified as salts, acids, or bases (3) ionic compounds are orderly, infinite arrangements of positive and negative ions (4) ionic compounds are built with foam bal ...
... (1) ionic compounds are never called molecules and have covalent bonds only in their polyatomic ions (2) ionic compounds are generally classified as salts, acids, or bases (3) ionic compounds are orderly, infinite arrangements of positive and negative ions (4) ionic compounds are built with foam bal ...
6.5-6.9 1 6.5 Quantum Mechanics and Atomic Orbitals
... magnetic quantum number determine(ml). What values can each of these quantum numbers have, how are their values related? What are the shapes of the orbitals for different values of the angular momentum quantum number (different subshells)? Sketch these shapes. What labels do we give these subshell ...
... magnetic quantum number determine(ml). What values can each of these quantum numbers have, how are their values related? What are the shapes of the orbitals for different values of the angular momentum quantum number (different subshells)? Sketch these shapes. What labels do we give these subshell ...
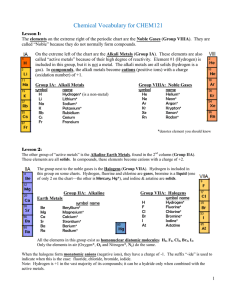
Vocabulary CHEM121
... Salts: all other ionic materials Formulas of molecular/covalent compounds: Non-metals can combine with other non-metals to form molecules. Molecules are covalently bonded groups of atoms—they do not have a charge like polyatomic ions, but are neutral. Since more than one combination is possible, the ...
... Salts: all other ionic materials Formulas of molecular/covalent compounds: Non-metals can combine with other non-metals to form molecules. Molecules are covalently bonded groups of atoms—they do not have a charge like polyatomic ions, but are neutral. Since more than one combination is possible, the ...
Stoichiometry – AP - Waukee Community School District Blogs
... Example Problem 3: Juglone, a dye known for centuries, is produced from the husks of black walnuts. It is also a natural herbicide that kills off competitive plants around the black walnut tree but does not affect grass and other noncompetitive plants. The formula for juglone is C10H6O3. (a.) Calcul ...
... Example Problem 3: Juglone, a dye known for centuries, is produced from the husks of black walnuts. It is also a natural herbicide that kills off competitive plants around the black walnut tree but does not affect grass and other noncompetitive plants. The formula for juglone is C10H6O3. (a.) Calcul ...
Chapter 2 Matter and Change
... either: 1) _________________ – the mixture is not uniform in composition • Chocolate chip cookie, gravel, soil. 2) __________________ - same composition throughout; called “solutions” • Kool-aid, air, salt water Every part keeps it’s own properties. ...
... either: 1) _________________ – the mixture is not uniform in composition • Chocolate chip cookie, gravel, soil. 2) __________________ - same composition throughout; called “solutions” • Kool-aid, air, salt water Every part keeps it’s own properties. ...
Energy Levels and Sub
... Because of observations made regarding ionization energy, the fine structure of element spectra, and new discoveries made regarding the wave nature of matter (specifically, electrons), scientists came up with a new model of the atom. In order to write a mathematical expression that would allow them ...
... Because of observations made regarding ionization energy, the fine structure of element spectra, and new discoveries made regarding the wave nature of matter (specifically, electrons), scientists came up with a new model of the atom. In order to write a mathematical expression that would allow them ...
Unit 1: Basic Chemistry for Biology QUIZ STUDY GUIDE Things to
... -You will see 12 of them on the quiz tomorrow. ...
... -You will see 12 of them on the quiz tomorrow. ...
Chemistry Unit Summaries - Oak Park Unified School District
... Molar mass (MM) is the sum of atomic masses in the chemical The electronic structure of an atom describes the energies formula. For example, the mass of one H2O molecule is 18.0 u, and arrangement of electrons around the atom. Much of what is so the molar mass of H2O is 18.0 g. known about the elect ...
... Molar mass (MM) is the sum of atomic masses in the chemical The electronic structure of an atom describes the energies formula. For example, the mass of one H2O molecule is 18.0 u, and arrangement of electrons around the atom. Much of what is so the molar mass of H2O is 18.0 g. known about the elect ...
16-1 and 16-2 Electric Charge
... electric field at a point some distance from two or more point charges. 6. Determine the magnitude and direction of the electric force on a charged particle placed in an electric field. 7. Sketch the electric field pattern in the region between charged objects. 8. Use Gauss's law to determine the ma ...
... electric field at a point some distance from two or more point charges. 6. Determine the magnitude and direction of the electric force on a charged particle placed in an electric field. 7. Sketch the electric field pattern in the region between charged objects. 8. Use Gauss's law to determine the ma ...
∙ ∙B x
... atoms at the corners of the tetrahedron. Because there are strong ....................... bonds that are difficult/easy to break between carbon atoms, diamond is very hard/soft, brittle/rigid, has low/high melting and boiling point. As all the valence electrons contribute to the bonding, they cannot ...
... atoms at the corners of the tetrahedron. Because there are strong ....................... bonds that are difficult/easy to break between carbon atoms, diamond is very hard/soft, brittle/rigid, has low/high melting and boiling point. As all the valence electrons contribute to the bonding, they cannot ...
Optically polarized atoms_ch_2_Atomic_States
... In this approximation, energy of a configuration is just sum of Ei No reference to projections of li or to spins degeneracy If we go beyond the central-field approximation some of the degeneracies will be lifted Also spin-orbit (ls) interaction lifts some degeneracies In general, both effects nee ...
... In this approximation, energy of a configuration is just sum of Ei No reference to projections of li or to spins degeneracy If we go beyond the central-field approximation some of the degeneracies will be lifted Also spin-orbit (ls) interaction lifts some degeneracies In general, both effects nee ...
A-Level Chemistry (A1) ATOMIC STRUCTURE
... Isotopes of an element have the same chemical properties because they have the same number of electrons. When a chemical reaction takes place, it is the electrons that are involved in the reactions. However isotopes of an element have the slightly different physical properties because they have diff ...
... Isotopes of an element have the same chemical properties because they have the same number of electrons. When a chemical reaction takes place, it is the electrons that are involved in the reactions. However isotopes of an element have the slightly different physical properties because they have diff ...
Regents Chemistry Topic Review Packet
... work of many scientists. Dalton’s Model: Elements are made of atoms Atoms of an element are the same. Compounds are formed from combinations of atoms. Rutherford Experiment Bombarded gold foil with alpha particles. Showed atoms were mostly empty space with small, dense positively charged ...
... work of many scientists. Dalton’s Model: Elements are made of atoms Atoms of an element are the same. Compounds are formed from combinations of atoms. Rutherford Experiment Bombarded gold foil with alpha particles. Showed atoms were mostly empty space with small, dense positively charged ...
Document
... Constant terms to be added. O(1) Table driven method used on lattices is O(N2). For N>1000 faster methods are known. ...
... Constant terms to be added. O(1) Table driven method used on lattices is O(N2). For N>1000 faster methods are known. ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.