Welcome to AP Chemistry!
... 2) Benzene contains only carbon and hydrogen and has a molar mass of 78.1 g/mol. Analysis shows the compound to be 7.74% H by mass. Find the empirical and molecular formulas of benzene. 3) Calcium carbonate decomposes upon heating, producing calcium oxide and carbon dioxide gas. a. Write a balanced ...
... 2) Benzene contains only carbon and hydrogen and has a molar mass of 78.1 g/mol. Analysis shows the compound to be 7.74% H by mass. Find the empirical and molecular formulas of benzene. 3) Calcium carbonate decomposes upon heating, producing calcium oxide and carbon dioxide gas. a. Write a balanced ...
File
... 45. Acetylene gas (C2H2) and Calcium hydroxide are produced by adding water to calcium carbide (CaC2). Write the balanced chemical equation. How many grams of acetylene are produced by adding excess water to 5.00 grams of calcium carbide? 46. Using the equation you balanced in the problem above, det ...
... 45. Acetylene gas (C2H2) and Calcium hydroxide are produced by adding water to calcium carbide (CaC2). Write the balanced chemical equation. How many grams of acetylene are produced by adding excess water to 5.00 grams of calcium carbide? 46. Using the equation you balanced in the problem above, det ...
Elements (NonMetals)
... Sulfides, selenides, tellurides only with group 1 and 2 metals are truly ionic Usually oxygen has negative oxidation number except F, S, Se, and Te have +4, +6 with O and Halogens ...
... Sulfides, selenides, tellurides only with group 1 and 2 metals are truly ionic Usually oxygen has negative oxidation number except F, S, Se, and Te have +4, +6 with O and Halogens ...
Lecture 31: The Hydrogen Atom 2: Dipole Moments Phy851 Fall 2009
... • The interaction between a hydrogen atom and an electric field is given to leading order by the Electric Dipole approximation: `Semi-Classical’ Approx: ...
... • The interaction between a hydrogen atom and an electric field is given to leading order by the Electric Dipole approximation: `Semi-Classical’ Approx: ...
paper - Center for Ultracold Atoms
... many-body system, which may facilitate the study of more exotic situations10. Two-particle correlation analysis is an increasingly important method for studying complex quantum phases of ultracold atoms7–13. It goes back to the discovery, by Hanbury Brown and Twiss1, that photons emitted by a chaoti ...
... many-body system, which may facilitate the study of more exotic situations10. Two-particle correlation analysis is an increasingly important method for studying complex quantum phases of ultracold atoms7–13. It goes back to the discovery, by Hanbury Brown and Twiss1, that photons emitted by a chaoti ...
IT IS ELEMENTARY - the OLLI at UCI Blog
... plant or animal origin—Sulfur was the exception • 1914-1918 A “golden age” for the military use of chemicals—the first weapons of mass destruction • Since the 1930s Chemical weapons have been used against defenseless peoples in colonial or civil wars ...
... plant or animal origin—Sulfur was the exception • 1914-1918 A “golden age” for the military use of chemicals—the first weapons of mass destruction • Since the 1930s Chemical weapons have been used against defenseless peoples in colonial or civil wars ...
PHYSICS OF THE ZERO-POINT FIELD: IMPLICATIONS FOR
... That matter should have the property of inertia is so fundamental to our conception of physical reality that it is difficult to imagine how matter could not have such an innate property. How could one have a stable, solid (including liquid and gas in this context) reality without inertia? Put anothe ...
... That matter should have the property of inertia is so fundamental to our conception of physical reality that it is difficult to imagine how matter could not have such an innate property. How could one have a stable, solid (including liquid and gas in this context) reality without inertia? Put anothe ...
Inert Gas Purification Systems
... • Restoration of the the Q5 is accomplished by passing a H2 rich gas through the hot reactant. The H2 reacts with the saturated reactant to form metallic Cu and H20. The water is then pumped out. ...
... • Restoration of the the Q5 is accomplished by passing a H2 rich gas through the hot reactant. The H2 reacts with the saturated reactant to form metallic Cu and H20. The water is then pumped out. ...
Review of Atomic Structure
... inform us that the electron appears to have only very specific or discrete values of energy. A further problem arises in regard to some other classical equations of physics (Maxwell’s equations) which have to do with moving charges and electromagnetic fields. These equations predict that the negativ ...
... inform us that the electron appears to have only very specific or discrete values of energy. A further problem arises in regard to some other classical equations of physics (Maxwell’s equations) which have to do with moving charges and electromagnetic fields. These equations predict that the negativ ...
Chapters 19 & 20
... decompose exothermically to the elements In the preparation of NH3 from N2 and H2, too much energy is needed to disrupt the N≡N bond. Thus, though K (106) is high the reaction is very slow at room temperature. Haber process is used to prepare NH3 (high pressure, high temperature and a catalyst are n ...
... decompose exothermically to the elements In the preparation of NH3 from N2 and H2, too much energy is needed to disrupt the N≡N bond. Thus, though K (106) is high the reaction is very slow at room temperature. Haber process is used to prepare NH3 (high pressure, high temperature and a catalyst are n ...
Field Evaporation of Grounded Arsenic Doped
... where r is the radius of the apex. 1/F is a linear function of L which agrees with Fig.2. C. J. Edgcombe et al. [18] calculated the field enhancement factor γ for various geometries and sizes of CNTs by means of the finite element method which demonstrated the similar 1/F~L relation. However, the di ...
... where r is the radius of the apex. 1/F is a linear function of L which agrees with Fig.2. C. J. Edgcombe et al. [18] calculated the field enhancement factor γ for various geometries and sizes of CNTs by means of the finite element method which demonstrated the similar 1/F~L relation. However, the di ...
Webquest Review - Harrison High School
... carbon. These two identical bonds in a symmetrical species, pull in equal yet opposite directions, cancelling each other out. E: View “Intermolecular Forces.” 20. Explain why magnesium chloride should dissolve in water. Magnesium chloride is an ionic compound composed of a cation and two anions. Mos ...
... carbon. These two identical bonds in a symmetrical species, pull in equal yet opposite directions, cancelling each other out. E: View “Intermolecular Forces.” 20. Explain why magnesium chloride should dissolve in water. Magnesium chloride is an ionic compound composed of a cation and two anions. Mos ...
Chapter 21: Electric Charge and Electric Field (01.03.2017)
... All materials are made up of atoms – building blocks of matter ‐ ...
... All materials are made up of atoms – building blocks of matter ‐ ...
Questions and Solutions
... K2Cr2O7 + 6NaI + 7H2SO4 → Cr2(SO4)3 + 3I2 + 7H2O + 3Na2SO4 + K2SO4 A 35.0 g sample of pure K2Cr2O7 produces 9.67 g of H2O. What is the percentage yield? ...
... K2Cr2O7 + 6NaI + 7H2SO4 → Cr2(SO4)3 + 3I2 + 7H2O + 3Na2SO4 + K2SO4 A 35.0 g sample of pure K2Cr2O7 produces 9.67 g of H2O. What is the percentage yield? ...
Chem 30A Fa_06 FE Review
... 160 Ci, what would be its activity after 24 days? How many days does it take for the activity to decrease to 5 Ci? (Answer: 20 Ci; 40 days) ...
... 160 Ci, what would be its activity after 24 days? How many days does it take for the activity to decrease to 5 Ci? (Answer: 20 Ci; 40 days) ...
Molecules
... the procession of these time varying fields, which mutually build one another as they propagate forward at the speed of light. The more energy the photon carries, the more concentrated these fields become—this does not make the electromagnetic (EM) wave travel faster, but it decreases it wavelength. ...
... the procession of these time varying fields, which mutually build one another as they propagate forward at the speed of light. The more energy the photon carries, the more concentrated these fields become—this does not make the electromagnetic (EM) wave travel faster, but it decreases it wavelength. ...
Quantum Electrodynamics
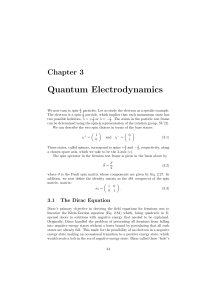
... among the greatest triumphs in theoretical physics. Later, Feynman came up with an alternative interpretation of positrons as electrons traveling backward in time. This led to great simplification of the theory, which came to be known as quantum electrodynamics. So, to modify the Klein-Gordon equati ...
... among the greatest triumphs in theoretical physics. Later, Feynman came up with an alternative interpretation of positrons as electrons traveling backward in time. This led to great simplification of the theory, which came to be known as quantum electrodynamics. So, to modify the Klein-Gordon equati ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.