IOSR Journal of Applied Physics (IOSR-JAP)
... application of Einstein’s field equations, with some success. Recently, some work on gauge symmetries in spinfoam gravity has been reported [10]. An interesting work on spin and quantization of gravitational space has also been reported [11]. Recently, there have been a lot of research activities to ...
... application of Einstein’s field equations, with some success. Recently, some work on gauge symmetries in spinfoam gravity has been reported [10]. An interesting work on spin and quantization of gravitational space has also been reported [11]. Recently, there have been a lot of research activities to ...
PHYSICAL SETTING CHEMISTRY
... 16 The kinetic molecular theory assumes that the particles of an ideal gas (1) are in random, constant, straight-line motion (2) are arranged in a regular geometric pattern (3) have strong attractive forces between them (4) have collisions that result in the system losing energy ...
... 16 The kinetic molecular theory assumes that the particles of an ideal gas (1) are in random, constant, straight-line motion (2) are arranged in a regular geometric pattern (3) have strong attractive forces between them (4) have collisions that result in the system losing energy ...
Chemistry 3211 – Coordination Chemistry Part 4 Electronic Spectra
... When white light strikes a substance, only specific wavelengths of light are absorbed since only specific quanta of energy are required to excite the electron based on the energy difference between the two states. Since any remaining frequencies of light are not absorbed, the substance reflects them ...
... When white light strikes a substance, only specific wavelengths of light are absorbed since only specific quanta of energy are required to excite the electron based on the energy difference between the two states. Since any remaining frequencies of light are not absorbed, the substance reflects them ...
NMR: Technical Background
... SOURCE E. P Steinberg, Johns Hopkins Medical Institutions, Baltimore. MD, 1983 ...
... SOURCE E. P Steinberg, Johns Hopkins Medical Institutions, Baltimore. MD, 1983 ...
A Quantum Similarity Study of Atomic Density Functions: Insights
... Quantification of molecular similarity using an electron density based Quantum Similarity Index (QSI) yields compact information on the similarity in shape and extent of the electron density distribution of various molecules. These data can be used as descriptor in comparative discussions of molecul ...
... Quantification of molecular similarity using an electron density based Quantum Similarity Index (QSI) yields compact information on the similarity in shape and extent of the electron density distribution of various molecules. These data can be used as descriptor in comparative discussions of molecul ...
Unit 1 Cycle 2: Interactions and Energy
... Your instructor will give you a copy of the handout Scientists' Ideas: Chemical Properties and Changes. Take a few minutes to review the Scientists' Ideas and make sure they correspond to the ideas the class has developed. In the space below each of the scientists’ ideas you should make a note of an ...
... Your instructor will give you a copy of the handout Scientists' Ideas: Chemical Properties and Changes. Take a few minutes to review the Scientists' Ideas and make sure they correspond to the ideas the class has developed. In the space below each of the scientists’ ideas you should make a note of an ...
Chapter 4 - Fredericksburg City Public Schools
... Another hypothesis by Glenn Seaborg is that element number 121 will start “the g block.” The “g” block will be another grouping, similar to the Lanthanides and Actinides, of 18 elements. I have a link on my website. Click on Seaborg’s Extended Periodic Table and take a look. Maybe your grandch ...
... Another hypothesis by Glenn Seaborg is that element number 121 will start “the g block.” The “g” block will be another grouping, similar to the Lanthanides and Actinides, of 18 elements. I have a link on my website. Click on Seaborg’s Extended Periodic Table and take a look. Maybe your grandch ...
Monday, March 8, 2010
... 1.0 kg mass that move apart at speeds of 0.6 c relative to original bomb. Find the original mass M. ...
... 1.0 kg mass that move apart at speeds of 0.6 c relative to original bomb. Find the original mass M. ...
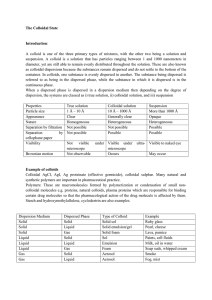
The Colloidal State Introduction: A colloid is one of the three primary
... particles in the dispersed phase can take place in different phases depending on how much water is available. For example, Jello powder mixed in with water creates a hydrocolloid. A common use for hydrocolloids is in the creation of medical dressings. In general colloids have the following propertie ...
... particles in the dispersed phase can take place in different phases depending on how much water is available. For example, Jello powder mixed in with water creates a hydrocolloid. A common use for hydrocolloids is in the creation of medical dressings. In general colloids have the following propertie ...
Mrs
... a. responsible for the outcome of their discoveries b. making scientific discoveries for science’s sake c. limited based on society’s moral values Chapter 17 True or False _____ 61. Electrons, protons, and neutrons combine to form atoms, which can be considered the building blocks of all matter. ___ ...
... a. responsible for the outcome of their discoveries b. making scientific discoveries for science’s sake c. limited based on society’s moral values Chapter 17 True or False _____ 61. Electrons, protons, and neutrons combine to form atoms, which can be considered the building blocks of all matter. ___ ...
Key III
... as being between the overlap of a(n) _ __ hybrid orbital on C with a(n) _ __ hybrid orbital on O. b) The sigma bonds formed between the hydrogen and carbon is best described as being the overlap of an __ _ hybrid orbital on each carbon with the _ __ orbital on the hydrogen atoms. c) The pi bond form ...
... as being between the overlap of a(n) _ __ hybrid orbital on C with a(n) _ __ hybrid orbital on O. b) The sigma bonds formed between the hydrogen and carbon is best described as being the overlap of an __ _ hybrid orbital on each carbon with the _ __ orbital on the hydrogen atoms. c) The pi bond form ...
O 95: Metal Substrates: Adsorption of Atoms and Inorganic Molecules
... In this study we combine quantitative LEED–IV, STM and DFT calculations to reinvestigate the adsorption behavior of oxygen on the Rh(100) surface in the coverage regime up to 0.67 ML. In this range three distinct phases exist: A (2 × 2)–O [1], a (2 × 2)–2O [2] and a (3×1)–2O structure [3]. The propo ...
... In this study we combine quantitative LEED–IV, STM and DFT calculations to reinvestigate the adsorption behavior of oxygen on the Rh(100) surface in the coverage regime up to 0.67 ML. In this range three distinct phases exist: A (2 × 2)–O [1], a (2 × 2)–2O [2] and a (3×1)–2O structure [3]. The propo ...
Chemical calculations review
... How many moles of oxygen are needed for the complete combustion of 3.0 moles of CH4(g)? 1. 6.0 moles ...
... How many moles of oxygen are needed for the complete combustion of 3.0 moles of CH4(g)? 1. 6.0 moles ...
m1 u q1 m2 q2 m3 q3 k1 k2
... m3 q̈3 + k2 (q3 − q2 ) = −dq̇3 . Indeed, the same result would have been obtained by Newton’s law (balance of forces) applied to the three masses. For the unforced (u = 0) equilibrium configurations, we set q̇ = q̈ = 0 in (2) and obtain an infinity of equilibria q 0 , all with equal q1 = q2 = q3 at ...
... m3 q̈3 + k2 (q3 − q2 ) = −dq̇3 . Indeed, the same result would have been obtained by Newton’s law (balance of forces) applied to the three masses. For the unforced (u = 0) equilibrium configurations, we set q̇ = q̈ = 0 in (2) and obtain an infinity of equilibria q 0 , all with equal q1 = q2 = q3 at ...
elements of chemistry unit
... Example 5. Aluminum and oxygen react to form aluminum oxide: 4 Al(s) + 3 O2(g) 2 Al2O3(s) 5a. Predict the before and after oxidation numbers for aluminum and oxygen. A. For Al: Aluminum is a pure element so it has a + 0 oxidation number. For O2: Oxygen is a pure element so it has a + 0 oxidation n ...
... Example 5. Aluminum and oxygen react to form aluminum oxide: 4 Al(s) + 3 O2(g) 2 Al2O3(s) 5a. Predict the before and after oxidation numbers for aluminum and oxygen. A. For Al: Aluminum is a pure element so it has a + 0 oxidation number. For O2: Oxygen is a pure element so it has a + 0 oxidation n ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.