fundamental_reality\Photons and Phonons
... variegated complexity that nevertheless cohere into a singular being. The wholeness of the organism is the ultimate problem of biocommunication: how to account for the continuity that encompasses the activities of elementary particles and atoms, molecules and cells, tissues and organs all the way to ...
... variegated complexity that nevertheless cohere into a singular being. The wholeness of the organism is the ultimate problem of biocommunication: how to account for the continuity that encompasses the activities of elementary particles and atoms, molecules and cells, tissues and organs all the way to ...
Practice Exam 3
... ____ 24. All of the following statements concerning nuclei are true EXCEPT a. only hydrogen-1 and helium-3 have more protons than neutrons. b. from He to Ca, stable nuclei have roughly equal numbers of protons and neutrons. c. isotopes with a low neutron to proton ratio always decay by alpha particl ...
... ____ 24. All of the following statements concerning nuclei are true EXCEPT a. only hydrogen-1 and helium-3 have more protons than neutrons. b. from He to Ca, stable nuclei have roughly equal numbers of protons and neutrons. c. isotopes with a low neutron to proton ratio always decay by alpha particl ...
The Quantum Jump Approach and Quantum Trajectories, Springer
... 5. Application to quantum arrival times An important open problem in quantum theory is the question of how to formulate the notion of “arrival time” of a particle, such as an atom, at a given location, i.e. the time instant of its first detection there. This is clearly a very physical question, but ...
... 5. Application to quantum arrival times An important open problem in quantum theory is the question of how to formulate the notion of “arrival time” of a particle, such as an atom, at a given location, i.e. the time instant of its first detection there. This is clearly a very physical question, but ...
Motion of atoms in a radiation trap
... working at sufficiently low saturation and with low dipole fluctuations, lifetimes of many seconds are possible for deep traps using reasonable opti- ...
... working at sufficiently low saturation and with low dipole fluctuations, lifetimes of many seconds are possible for deep traps using reasonable opti- ...
Arenes - Science Skool!
... A little dinitrobenzene may also be formed by the further attack of NO2+ on nitrobenzene (which is now more suspectible to attack due to the electron inductive effect of the NO2+ group). This extra group will go the the 3 position to give the disubstituted product 1,3-dinitrobenzene. To actually pro ...
... A little dinitrobenzene may also be formed by the further attack of NO2+ on nitrobenzene (which is now more suspectible to attack due to the electron inductive effect of the NO2+ group). This extra group will go the the 3 position to give the disubstituted product 1,3-dinitrobenzene. To actually pro ...
Special Theory of Relativity
... high speed, another theory was needed. • This is where Einstein stepped in. • In 1915 Einstein published his general theory of relativity. – Even though this theory is what Einstein is mostly known for, it is not what won him a Nobel Prize – His Nobel Prize was for his explanation of the Photoelectr ...
... high speed, another theory was needed. • This is where Einstein stepped in. • In 1915 Einstein published his general theory of relativity. – Even though this theory is what Einstein is mostly known for, it is not what won him a Nobel Prize – His Nobel Prize was for his explanation of the Photoelectr ...
File
... • The formula masses have a similar relationship. x(empirical formula mass) = molecular formula mass • To determine the molecular formula of a compound, you must know the compound’s formula mass. • Dividing the experimental formula mass by the empirical formula mass gives the value of x. ...
... • The formula masses have a similar relationship. x(empirical formula mass) = molecular formula mass • To determine the molecular formula of a compound, you must know the compound’s formula mass. • Dividing the experimental formula mass by the empirical formula mass gives the value of x. ...
The Mole
... Mr(Li2CO3) = 73.89 g/mol 2) Calculate the amount of Li2CO3 in moles: n (Li2CO3) = 0.359 mol 3) Calculate the amount of O atoms in moles: n (O) = (0.359x3) mol = 1.077 mol 4) Multiply by the Avogadro’s number to obtain the answer: N (O) = 1.077 mol x 6.022·1023 atom/mol = = 6.50·1023 atoms ...
... Mr(Li2CO3) = 73.89 g/mol 2) Calculate the amount of Li2CO3 in moles: n (Li2CO3) = 0.359 mol 3) Calculate the amount of O atoms in moles: n (O) = (0.359x3) mol = 1.077 mol 4) Multiply by the Avogadro’s number to obtain the answer: N (O) = 1.077 mol x 6.022·1023 atom/mol = = 6.50·1023 atoms ...
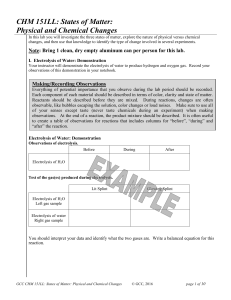
CHM 151LL: States of Matter: Physical and Chemical Changes
... Substances can exist in three physical states: solid, liquid, and gas. Some of the differences between these states of matter are 1) the atoms’ or molecules’ freedom of movement and 2) the amount of space between the atoms or molecules. The physical state of a substance at a specific temperature dep ...
... Substances can exist in three physical states: solid, liquid, and gas. Some of the differences between these states of matter are 1) the atoms’ or molecules’ freedom of movement and 2) the amount of space between the atoms or molecules. The physical state of a substance at a specific temperature dep ...
Chapter One
... 1) Calculate the molar mass of Li2CO3: Mr(Li2CO3) = 73.89 g/mol 2) Calculate the amount of Li2CO3 in moles: n (Li2CO3) = 0.359 mol 3) Calculate the amount of O atoms in moles: n (O) = (0.359x3) mol = 1.077 mol 4) Multiply by the Avogadro’s number to obtain the answer: N (O) = 1.077 mol x 6.022·1023 ...
... 1) Calculate the molar mass of Li2CO3: Mr(Li2CO3) = 73.89 g/mol 2) Calculate the amount of Li2CO3 in moles: n (Li2CO3) = 0.359 mol 3) Calculate the amount of O atoms in moles: n (O) = (0.359x3) mol = 1.077 mol 4) Multiply by the Avogadro’s number to obtain the answer: N (O) = 1.077 mol x 6.022·1023 ...
K = 1 2 mv W = Fds ︷︸︸︷ = Fd ΑK = K −Ki =W
... m1 = 0.5 kg, the speed of the hammer head when it strikes the nail is v1 = 200 m/s. The head then bounces back with a speed of v2 = 100 m/s (opposite direction). The nails mass is m2 = 5g. If the frictional force between the nail and the wood is Ff = 104N, how far will the nail go into the wood? Ste ...
... m1 = 0.5 kg, the speed of the hammer head when it strikes the nail is v1 = 200 m/s. The head then bounces back with a speed of v2 = 100 m/s (opposite direction). The nails mass is m2 = 5g. If the frictional force between the nail and the wood is Ff = 104N, how far will the nail go into the wood? Ste ...
A Bell Theorem Without Inequalities for Two
... met are suddenly thrown into an entanglement, even though they have no shared history. We will show that the situation is strange enough so that one cannot reproduce the quantum perfect correlations of the entanglement-swapped state with a classical, deterministic theory. While the original experime ...
... met are suddenly thrown into an entanglement, even though they have no shared history. We will show that the situation is strange enough so that one cannot reproduce the quantum perfect correlations of the entanglement-swapped state with a classical, deterministic theory. While the original experime ...
SPH3U Final Exam Review
... 1. You are travelling in a spacecraft headed straight toward the Moon. Describe an experiment you could perform to determine whether the craft is travelling at constant speed or accelerating. 2. Suppose two astronauts synchronize their clocks and travel through space on different ships. When they ar ...
... 1. You are travelling in a spacecraft headed straight toward the Moon. Describe an experiment you could perform to determine whether the craft is travelling at constant speed or accelerating. 2. Suppose two astronauts synchronize their clocks and travel through space on different ships. When they ar ...
Chapter 3 Stoichiometry: Calculations with Chemical
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
Marking Scheme - The Physics Teacher
... Note to teachers and students on the use of published marking schemes Marking schemes published by the State Examinations Commission are not intended to be standalone documents. They are an essential resource for examiners who receive training in the correct interpretation and application of the sc ...
... Note to teachers and students on the use of published marking schemes Marking schemes published by the State Examinations Commission are not intended to be standalone documents. They are an essential resource for examiners who receive training in the correct interpretation and application of the sc ...
Stoichiometry, % Comp, Empirical & Molecular Formula
... product STOICHIOMETRY allows us to determine the other quantities. We use conversion factors from: ...
... product STOICHIOMETRY allows us to determine the other quantities. We use conversion factors from: ...
Chapter 2 Power Point
... KC: How can Properties be used to describe matter? A: Properties used to describe matter can be classified as ether extensive or intensive. ...
... KC: How can Properties be used to describe matter? A: Properties used to describe matter can be classified as ether extensive or intensive. ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.