Contents
... Almost everything we utilize today has been produced by means of a chemical reaction. For example, the shells of electronic devices are usually made of plastic. The plastic was synthesized from a chemical compound which itself had been produced from oil. The silicon at the heart of most electronic d ...
... Almost everything we utilize today has been produced by means of a chemical reaction. For example, the shells of electronic devices are usually made of plastic. The plastic was synthesized from a chemical compound which itself had been produced from oil. The silicon at the heart of most electronic d ...
Space For Refection
... In this case the light rays are slowed down by the water and are _____, causing the pen to look odd. The two mediums in this example are ______ and _______. The amount of refraction that a medium will do can be measured by its “refractive index” – the higher the index, the more refractive the medium ...
... In this case the light rays are slowed down by the water and are _____, causing the pen to look odd. The two mediums in this example are ______ and _______. The amount of refraction that a medium will do can be measured by its “refractive index” – the higher the index, the more refractive the medium ...
Nonlinear Schrödinger equation and
... Let us consider a hydrogen atom that is in a light field and account for its spontaneous emission. Under the action of the light field, the excitation of some eigenmodes of the electron wave occurs in the hydrogen atom at the expense of others. This phenomenon will be manifested in a stimulated redist ...
... Let us consider a hydrogen atom that is in a light field and account for its spontaneous emission. Under the action of the light field, the excitation of some eigenmodes of the electron wave occurs in the hydrogen atom at the expense of others. This phenomenon will be manifested in a stimulated redist ...
Course Pack3 Phase Diagrams
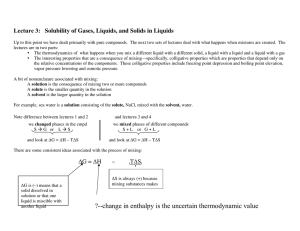
... ∆G is (–) means that a solid dissolved in solution or that one liquid is miscible with ...
... ∆G is (–) means that a solid dissolved in solution or that one liquid is miscible with ...
Quantum Mechanics - University of Colorado Boulder
... Each of our theories, except relativistic Quantum Mechanics, has a limited regime of validity. As far as we can tell (to date), QM (relativistic version) is perfectly correct. It works for all situations, no matter how small or how fast. Well... this is not quite true: no one knows how to properly d ...
... Each of our theories, except relativistic Quantum Mechanics, has a limited regime of validity. As far as we can tell (to date), QM (relativistic version) is perfectly correct. It works for all situations, no matter how small or how fast. Well... this is not quite true: no one knows how to properly d ...
Separated spin-up and spin-down evolution of degenerated
... Fig. (5) describes the SEAW in the cylindric 2DEG for l=0, 1, 2, 3. General behavior shows resemblance to dispersion of the SEAW in plane-like 2DEG: increase of ξ(k), lowering of ξ(k) with increasing of spin polarisation η. Increasing of l reveals in modification of dispersion surface of the SEAW. V ...
... Fig. (5) describes the SEAW in the cylindric 2DEG for l=0, 1, 2, 3. General behavior shows resemblance to dispersion of the SEAW in plane-like 2DEG: increase of ξ(k), lowering of ξ(k) with increasing of spin polarisation η. Increasing of l reveals in modification of dispersion surface of the SEAW. V ...
Chapter 4 Quantities of Reactants and Products 4.1 Chemical
... Combustion Analysis Problem #1 Benzene is a liquid compound composed of carbon and hydrogen. A sample of benzene weighing 342 mg is burned in excess oxygen and forms 1156 mg of carbon dioxide. What is the percent composition of benzene? [92.25%C & 7.75% H] Combustion Analysis Problem #2 n-Butyl phth ...
... Combustion Analysis Problem #1 Benzene is a liquid compound composed of carbon and hydrogen. A sample of benzene weighing 342 mg is burned in excess oxygen and forms 1156 mg of carbon dioxide. What is the percent composition of benzene? [92.25%C & 7.75% H] Combustion Analysis Problem #2 n-Butyl phth ...
Evade the Heisenberg Uncertainty Principle
... Consequently, in 2011, a novel, more direct tomographical method was established to determine the quantum state without the need for post-processing. However, that novel method had a major drawback: It uses minimally disturbing measurements, so-called weak measurements, to determine the system's qua ...
... Consequently, in 2011, a novel, more direct tomographical method was established to determine the quantum state without the need for post-processing. However, that novel method had a major drawback: It uses minimally disturbing measurements, so-called weak measurements, to determine the system's qua ...
A Model of the Chemical Bond Must Be Rooted in Quantum
... and argue about molecular structure and stability. The boost that chemistry can receive from such insight has been demonstrated by the work of Roald Hoffmann and his school.[12] Thus, the concepts and quantities of the model must be of such a nature that one can rationally and qualitatively predict ...
... and argue about molecular structure and stability. The boost that chemistry can receive from such insight has been demonstrated by the work of Roald Hoffmann and his school.[12] Thus, the concepts and quantities of the model must be of such a nature that one can rationally and qualitatively predict ...
Stoichiometry
... What the mass of H2 must be supplied to make 500. kg of NH3 in a system that has an 88.8% yield? Percent yield = 100% x actual yield/theore?cal yield Theore?cal yield = 100% x actual yield/percent yield = 100% x 500. kg of NH3 / 88.8% = 563 kg of NH3 563,000 gm NH3 x 1 mole NH3 x 3 ...
... What the mass of H2 must be supplied to make 500. kg of NH3 in a system that has an 88.8% yield? Percent yield = 100% x actual yield/theore?cal yield Theore?cal yield = 100% x actual yield/percent yield = 100% x 500. kg of NH3 / 88.8% = 563 kg of NH3 563,000 gm NH3 x 1 mole NH3 x 3 ...
std 6 review12ans
... Standard 6a: Students know the definitions of solute and solvent. Match the following: 1. The chemical that is doing the dissolving (there is more of it) in the solution. b ...
... Standard 6a: Students know the definitions of solute and solvent. Match the following: 1. The chemical that is doing the dissolving (there is more of it) in the solution. b ...
Unit - 7.pmd
... Similarly, in case of phosphorus nearly all intermediate oxidation states disproportionate into +5 and –3 both in alkali and acid. However +3 oxidation state in case of arsenic, antimony and bismuth becomes increasingly stable with respect to disproportionation. Nitrogen is restricted to a maximum c ...
... Similarly, in case of phosphorus nearly all intermediate oxidation states disproportionate into +5 and –3 both in alkali and acid. However +3 oxidation state in case of arsenic, antimony and bismuth becomes increasingly stable with respect to disproportionation. Nitrogen is restricted to a maximum c ...
Introduction to the Weak Interaction, Volume 1
... 4T(x, t) are, in general, complex . Hence Born suggested that the probability should be the product of the value of the wave function and its complex conjugate , so that the probability of finding the particle between x and x + dx is given by P(x, t) dx ...
... 4T(x, t) are, in general, complex . Hence Born suggested that the probability should be the product of the value of the wave function and its complex conjugate , so that the probability of finding the particle between x and x + dx is given by P(x, t) dx ...
Prediction of a quantum anomalous Hall state in Co decorated silicene
... in the irreducible Brillouin zone is used. Silicene has been demonstrated to exist on various substrates. While we do not take into account a specific substrate in our calculations, our results are valid not only for a suspended sample but also in the case that the interaction with the substrate is ...
... in the irreducible Brillouin zone is used. Silicene has been demonstrated to exist on various substrates. While we do not take into account a specific substrate in our calculations, our results are valid not only for a suspended sample but also in the case that the interaction with the substrate is ...
Redox Reactions - Hillsborough County Public Schools
... A compound has an overall charge of zero, which means all the negative charges have to equal the positive charges. Examples: When calculating the oxidation number of N in NO2 , use the rules above to help you. You see that oxygen normally has an oxidation number of -2 and there are two oxygen atoms. ...
... A compound has an overall charge of zero, which means all the negative charges have to equal the positive charges. Examples: When calculating the oxidation number of N in NO2 , use the rules above to help you. You see that oxygen normally has an oxidation number of -2 and there are two oxygen atoms. ...
Contents - L`esperimento più bello della fisica
... experiment. It then looks at nature on a quantum scale by investigating the behaviour of electrons in the double-slit experiment. This experiment yields results that defy classical thinking. Electrons leave the source as particles and strike the detection screen as particles, producing small localiz ...
... experiment. It then looks at nature on a quantum scale by investigating the behaviour of electrons in the double-slit experiment. This experiment yields results that defy classical thinking. Electrons leave the source as particles and strike the detection screen as particles, producing small localiz ...
Notes on QA - Scarsdale Public Schools
... Notes on Qualitative Analysis Qualitative analysis involves separating a mixture of cations based on their solubilities. In the traditional QA scheme, the Group 1 cations (not the periodic table group 1) are separated from a mixture of dozens of cations based on their insolubility as chloride salts. ...
... Notes on Qualitative Analysis Qualitative analysis involves separating a mixture of cations based on their solubilities. In the traditional QA scheme, the Group 1 cations (not the periodic table group 1) are separated from a mixture of dozens of cations based on their insolubility as chloride salts. ...
Paper
... to study many-body phenomena because of the relative ease with which parameters in the model Hamiltonian can be tuned across a wide range [1,2]. Such studies have resulted in a better understanding of various phase transitions such as the Berezinskii-Kosterlitz-Thouless transition in two-dimensional ...
... to study many-body phenomena because of the relative ease with which parameters in the model Hamiltonian can be tuned across a wide range [1,2]. Such studies have resulted in a better understanding of various phase transitions such as the Berezinskii-Kosterlitz-Thouless transition in two-dimensional ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.