![chap15pptlecture_chapte.ppt [Read-Only]](http://s1.studyres.com/store/data/015369082_1-00cbf06a2d468a4ae1c963f5ca674e31-300x300.png)
b - Gordon State College
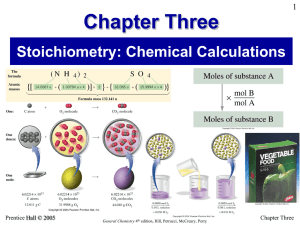
... 2) Find the moles of each reactant: moles = mass in gram / molar mass 3) Pick up any reactant, say A, and use the stoichiometry to calculate the required amount of the other reactant B. 4) Compare the required amount of B with the available amount of B. a) If required > available, then B is the limi ...
... 2) Find the moles of each reactant: moles = mass in gram / molar mass 3) Pick up any reactant, say A, and use the stoichiometry to calculate the required amount of the other reactant B. 4) Compare the required amount of B with the available amount of B. a) If required > available, then B is the limi ...
Test
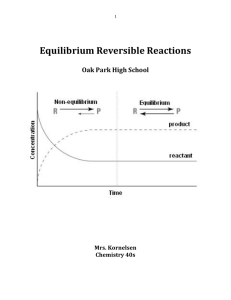
... The concentration(s) of the reactant(s) is equal to the concentration(s) of the product(s). b) No new product molecules are formed. c) The concentration(s) of reactant(s) is constant over time. d) The rate of the reverse reaction is equal to the rate of the forward reaction and both rates are equal ...
... The concentration(s) of the reactant(s) is equal to the concentration(s) of the product(s). b) No new product molecules are formed. c) The concentration(s) of reactant(s) is constant over time. d) The rate of the reverse reaction is equal to the rate of the forward reaction and both rates are equal ...
Chapter Three
... reactants products in a chemical reaction are represented by words. • Remember – what are our diatomic elements? • Ex#1: Solid aluminum reacts with gaseous oxygen to produce solid aluminum oxide. • Ex#2: Methane, in the presence of oxygen, combusts into water and carbon dioxide. Prentice Hall © 2005 ...
... reactants products in a chemical reaction are represented by words. • Remember – what are our diatomic elements? • Ex#1: Solid aluminum reacts with gaseous oxygen to produce solid aluminum oxide. • Ex#2: Methane, in the presence of oxygen, combusts into water and carbon dioxide. Prentice Hall © 2005 ...
Chapter 1: Matter and Change
... Matter in the liquid state has a definite volume but an indefinite shape; a liquid assumes the shape of its container. For example, a given quantity of liquid water takes up a definite amount of space, but the water takes the shape of its container. Liquids have this characteristic because the parti ...
... Matter in the liquid state has a definite volume but an indefinite shape; a liquid assumes the shape of its container. For example, a given quantity of liquid water takes up a definite amount of space, but the water takes the shape of its container. Liquids have this characteristic because the parti ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... Aqueous Reactions and Solution Stoichiometry Aqueous Reactions ...
... Aqueous Reactions and Solution Stoichiometry Aqueous Reactions ...
Document
... 1. The concentrations of the reacting species in the condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm. 2. The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. 3. The equilibrium ...
... 1. The concentrations of the reacting species in the condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm. 2. The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. 3. The equilibrium ...
chapter_14 Equilibr
... 1. The concentrations of the reacting species in the condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm. 2. The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. 3. The equilibrium ...
... 1. The concentrations of the reacting species in the condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm. 2. The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. 3. The equilibrium ...
Practice Problem - HCC Southeast Commons
... – They are the “same” if the positions of the atoms can coincide on a one-to-one basis • We test if they are superimposable, which is imaginary ...
... – They are the “same” if the positions of the atoms can coincide on a one-to-one basis • We test if they are superimposable, which is imaginary ...
1. Atomic Structure and Periodic Table THE MASS SPECTROMETER
... If a molecule is put through a mass spectrometer it will often break up and give a series of peaks caused by the fragments. The peak with the largest m/z, however, will be due to the complete molecule and will be equal to the Mr of the molecule. This peak is called the parent ion or molecular ion ...
... If a molecule is put through a mass spectrometer it will often break up and give a series of peaks caused by the fragments. The peak with the largest m/z, however, will be due to the complete molecule and will be equal to the Mr of the molecule. This peak is called the parent ion or molecular ion ...
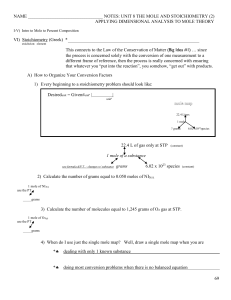
NAME NOTES: UNIT 8 THE MOLE AND STOICHIOMETRY (2
... 1) First, the coefficients of the balanced equation represent the mole ratios between each of the reactants, each of the products and each reactant to each product. 2) The coefficients tend to be in the simplest whole number ratio 3) Many chemistry teachers approach the balanced equation using a rec ...
... 1) First, the coefficients of the balanced equation represent the mole ratios between each of the reactants, each of the products and each reactant to each product. 2) The coefficients tend to be in the simplest whole number ratio 3) Many chemistry teachers approach the balanced equation using a rec ...
Chapter 4
... 1. Identify the species present in the combined solution, and determine what reaction if any occurs. 2. Write the balanced net ionic equation for the reaction. 3. Calculate the moles of reactants. 4. Determine which reactant is limiting. 5. Calculate the moles of product(s), as required. 6. Convert ...
... 1. Identify the species present in the combined solution, and determine what reaction if any occurs. 2. Write the balanced net ionic equation for the reaction. 3. Calculate the moles of reactants. 4. Determine which reactant is limiting. 5. Calculate the moles of product(s), as required. 6. Convert ...
PowerPoint Presentation - Chemical Equilibrium
... B) is correct assuming that 1.0 – X can be approximated to 1.0. The relatively small value for K indicates that, compared to 1.0, X would not be large enough to include in the calculation. The equilibrium expression could be simplified to K = X2/1.0. A quick test of this hypothesis could be made by ...
... B) is correct assuming that 1.0 – X can be approximated to 1.0. The relatively small value for K indicates that, compared to 1.0, X would not be large enough to include in the calculation. The equilibrium expression could be simplified to K = X2/1.0. A quick test of this hypothesis could be made by ...
chemistry
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
Porous silicon-based nanostructured microparticles as degradable
... 1(3)): A solution of 11-undecenylamine (0.677 g, 4 mmol) in 30 mL of anhydrous CH2Cl2 was prepared and cooled in an ice bath. In a separate flask, 9-fluorenylmethoxycarbonyl chloride (1.04 g, 4 mmol) was dissolved in a small amount of anhydrous CH2Cl2 and was slowly added to the solution of 11undece ...
... 1(3)): A solution of 11-undecenylamine (0.677 g, 4 mmol) in 30 mL of anhydrous CH2Cl2 was prepared and cooled in an ice bath. In a separate flask, 9-fluorenylmethoxycarbonyl chloride (1.04 g, 4 mmol) was dissolved in a small amount of anhydrous CH2Cl2 and was slowly added to the solution of 11undece ...
total review package - Lighthouse Christian Academy
... Write a balanced chemical equation for each of the following, and classify each as synthesis, decomposition, single replacement, double replacement, neutralization or combustion. a) ...
... Write a balanced chemical equation for each of the following, and classify each as synthesis, decomposition, single replacement, double replacement, neutralization or combustion. a) ...
Stoichiometry

Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.