Chemistry Semester 2 Final Exam Chemistry Semester 2 Final Exam
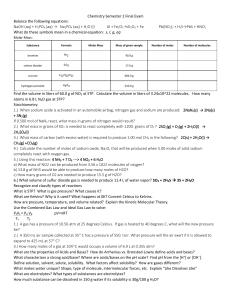
... 3.) What mass of carbon (with excess water) is required to produce 1.00 mol CH4 in the following? 2C(s) + 2H2O(l) → CH4(g) +CO2(g) 24.02 g 4.) Calculate the number of moles of sodium oxide, Na2O, that will be produced when 5.00 moles of solid sodium completely react with oxygen gas. 2.5 mol 5.) Usin ...
... 3.) What mass of carbon (with excess water) is required to produce 1.00 mol CH4 in the following? 2C(s) + 2H2O(l) → CH4(g) +CO2(g) 24.02 g 4.) Calculate the number of moles of sodium oxide, Na2O, that will be produced when 5.00 moles of solid sodium completely react with oxygen gas. 2.5 mol 5.) Usin ...
Exam Review – Part 1
... Hydrocarbons are molecules that contain only carbon and hydrogen They are made from fossil fuels (e.g. methane, propane, octane) and contain large amount of energy If enough oxygen is present, they will burn completely and release all of their energy and produce only two products: carbon dioxi ...
... Hydrocarbons are molecules that contain only carbon and hydrogen They are made from fossil fuels (e.g. methane, propane, octane) and contain large amount of energy If enough oxygen is present, they will burn completely and release all of their energy and produce only two products: carbon dioxi ...
Review Package
... Naming & writing formula - binary molecular compounds 7. Conservation of Mass & Chemical Equations (Textbook p. 159-168) Terminology (reactant, product, chemical reaction, chemical equation, coefficient) Law of Conservation of Mass Writing word equations, skeleton equations, balanced chemica ...
... Naming & writing formula - binary molecular compounds 7. Conservation of Mass & Chemical Equations (Textbook p. 159-168) Terminology (reactant, product, chemical reaction, chemical equation, coefficient) Law of Conservation of Mass Writing word equations, skeleton equations, balanced chemica ...
19a - The BOD
... “Dissolved oxygen” is there. It is a measure of how much oxygen is dissolved in a water sample. It is a measure of oxygen content. BOD is the amount of oxygen that would be consumed to completely decompose the organic matter in a water sample. It is not an indication of oxygen content. It is an indi ...
... “Dissolved oxygen” is there. It is a measure of how much oxygen is dissolved in a water sample. It is a measure of oxygen content. BOD is the amount of oxygen that would be consumed to completely decompose the organic matter in a water sample. It is not an indication of oxygen content. It is an indi ...
General, Organic, and Biological Chemistry
... 46) Isotopes are atoms of the same element that have A) different atomic numbers. B) the same atomic numbers but different numbers of protons. C) the same atomic numbers but different numbers of electrons. D) the same atomic number but different numbers of neutrons. E) the same atomic mass but diff ...
... 46) Isotopes are atoms of the same element that have A) different atomic numbers. B) the same atomic numbers but different numbers of protons. C) the same atomic numbers but different numbers of electrons. D) the same atomic number but different numbers of neutrons. E) the same atomic mass but diff ...
FXM Rev 1 Key - Grande Cache Community High School
... hydrogen bond This type of bond forms due to the electrostatic attraction between the positive end of a hydrogen atom with negative oxygen end in molecules. precipitate This is a solid material that may form as a result of a chemical reaction when the product is less soluble that the reactant. produ ...
... hydrogen bond This type of bond forms due to the electrostatic attraction between the positive end of a hydrogen atom with negative oxygen end in molecules. precipitate This is a solid material that may form as a result of a chemical reaction when the product is less soluble that the reactant. produ ...
chapter3 - AlvarezHChem
... example – CO2, CH4, etc. Formula unit – the representative particle for an ionic compound (metal and non-metal or polyatomic ion) ...
... example – CO2, CH4, etc. Formula unit – the representative particle for an ionic compound (metal and non-metal or polyatomic ion) ...
GC97F Pretest A - American Chemical Society
... 32. If the reaction is at equilibrium with excess C(s) remaining, what change will increase the quantity of CO(g) for the reaction at equilibrium? I Adding C(s) II Increasing the temperature III Increasing the pressure (A) I only ...
... 32. If the reaction is at equilibrium with excess C(s) remaining, what change will increase the quantity of CO(g) for the reaction at equilibrium? I Adding C(s) II Increasing the temperature III Increasing the pressure (A) I only ...
Chemistry Curriculum Guide
... AVOGADRO’S PRINCIPLE AND MOLAR VOLUME; STOICHIOMETRIC RELATIONSHIPS ...
... AVOGADRO’S PRINCIPLE AND MOLAR VOLUME; STOICHIOMETRIC RELATIONSHIPS ...
Name - Net Start Class
... 3. Define ‘valance electron’ and tell how many valance electrons each of the following elements have. a. Definition – electrons in the outermost energy level of an atom. These are the electrons involved in chemical reactions. b. Silicon 4 c. Chlorine 7 d. Magnesium 2 e. Krypton 8 4. How do you calcu ...
... 3. Define ‘valance electron’ and tell how many valance electrons each of the following elements have. a. Definition – electrons in the outermost energy level of an atom. These are the electrons involved in chemical reactions. b. Silicon 4 c. Chlorine 7 d. Magnesium 2 e. Krypton 8 4. How do you calcu ...
Chemical Equations I
... needed to “use up” the LR • Step 4: Subtract amount needed from amount available ...
... needed to “use up” the LR • Step 4: Subtract amount needed from amount available ...
CHEM MINI-COURSE SERIES M1.2___
... each type of atoms must appear on both sides of an equation. The atoms merely rearrange or regroup into different elements or compounds; they will not change into other atoms or be lost through a chemical reaction. For example, hydrogen gas and oxygen gas react to produce water. The following equati ...
... each type of atoms must appear on both sides of an equation. The atoms merely rearrange or regroup into different elements or compounds; they will not change into other atoms or be lost through a chemical reaction. For example, hydrogen gas and oxygen gas react to produce water. The following equati ...
Glossary (PDF file)
... experiment that is changed. In the plant experiment (see definition for constant), the independent variable is the amount of water the plants receive. This factor is the only one that changed during the experiment. indicator A substance that changes color with a change in pH. Phenol red is an example ...
... experiment that is changed. In the plant experiment (see definition for constant), the independent variable is the amount of water the plants receive. This factor is the only one that changed during the experiment. indicator A substance that changes color with a change in pH. Phenol red is an example ...
Unit D: Quantitative Relationships in Chemical Change
... and a precipitate forms. What amount of precipitate will form if the student has reacted 0.314 mol of silver nitrate? ...
... and a precipitate forms. What amount of precipitate will form if the student has reacted 0.314 mol of silver nitrate? ...
L22 - Supplementary Student Notes Package
... c) What mass of sulfur must have reacted in order to produce 75 g of the compound? ...
... c) What mass of sulfur must have reacted in order to produce 75 g of the compound? ...
Unit 11: The Mole
... Mass of element X 100 = Percent by Mass Mass of compound Examples: Sodium hydrogen carbonate, also called baking soda, is an active ingredient in some antacids used for the relief of indigestion. Determine the percent composition of sodium hydrogen carbonate. ...
... Mass of element X 100 = Percent by Mass Mass of compound Examples: Sodium hydrogen carbonate, also called baking soda, is an active ingredient in some antacids used for the relief of indigestion. Determine the percent composition of sodium hydrogen carbonate. ...
Chemical Equations and Reactions
... or more substances are changed into one or more different substances. • In any chemical reaction, the original substances are known as the reactants and the resulting substances are known as the products. • According to the law of conservation of mass, the total mass of reactants must equal the tota ...
... or more substances are changed into one or more different substances. • In any chemical reaction, the original substances are known as the reactants and the resulting substances are known as the products. • According to the law of conservation of mass, the total mass of reactants must equal the tota ...
Calculations with Chemical Formulas and Equations
... Molar Mass The trick: • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... Molar Mass The trick: • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
Balancing ANY chemical Equation
... A new substance is created. Bonds that hold the atoms together are broken and new bonds form. Indications of a Chemical Reaction: 1. Production of Heat and/or Light 2. Production of a Gas ( CO2 , H2 ) 3. Precipitate (solid in a liquid) forms. We use balanced chemical equations to REPRESENT a chemica ...
... A new substance is created. Bonds that hold the atoms together are broken and new bonds form. Indications of a Chemical Reaction: 1. Production of Heat and/or Light 2. Production of a Gas ( CO2 , H2 ) 3. Precipitate (solid in a liquid) forms. We use balanced chemical equations to REPRESENT a chemica ...
Chap 2.1 Notes - Nature of Matter
... pH – is a measure of the acidity or alkalinity of a solution. ...
... pH – is a measure of the acidity or alkalinity of a solution. ...
Stoichiometry

Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.