Reaction of amino acids with exo-3,6-epoxy-1,2,3,6
... material which corresponded with that reported by them.6 However, upon attempted crystallization from chloroform, as previously reported for 1 by Rich et al.5 it became apparent that the isolated material was not N-maleoylglycine 1. The material was instead crystallized from methanol-chloroform and ...
... material which corresponded with that reported by them.6 However, upon attempted crystallization from chloroform, as previously reported for 1 by Rich et al.5 it became apparent that the isolated material was not N-maleoylglycine 1. The material was instead crystallized from methanol-chloroform and ...
Writing Chemical Reactions
... Combination reactions can refer to the very simple reaction of two elements (a redox process) to form a compound. However, some compounds also combine to form other compounds. These are sometimes less obvious. In the former case, the reaction is mostly challenging because the correct formula of the ...
... Combination reactions can refer to the very simple reaction of two elements (a redox process) to form a compound. However, some compounds also combine to form other compounds. These are sometimes less obvious. In the former case, the reaction is mostly challenging because the correct formula of the ...
Final "I Can Statements" Answer Key
... percent composition of an element in a compound. _____24. I can convert between moles and numbers of particles using Avogadro’s number? _____25. I can convert between moles and L (assuming STP). ...
... percent composition of an element in a compound. _____24. I can convert between moles and numbers of particles using Avogadro’s number? _____25. I can convert between moles and L (assuming STP). ...
updated chem cp final review key
... x Moles HCl 6. Given the equation: __2_ Na + __2__ H2O _2___ NaOH + ____ H2 According to the following reaction, calculate the number of grams of sodium hydroxide that will be produced if 8.215 grams of sodium are reacted with excess water. Step 1: Convert grams to Moles 8.215 g Na × 1 Mole Na = 0 ...
... x Moles HCl 6. Given the equation: __2_ Na + __2__ H2O _2___ NaOH + ____ H2 According to the following reaction, calculate the number of grams of sodium hydroxide that will be produced if 8.215 grams of sodium are reacted with excess water. Step 1: Convert grams to Moles 8.215 g Na × 1 Mole Na = 0 ...
Mole calculations File
... A hydrocarbon contains 5.00g carbon and 1.25g hydrogen. One mole of the hydrocarbon has a mass of 30.00g 1. Calculate amount of each element amount (mol) = mass/molar mass amount C = 5.00/12.0 = 0.417mol amount H = 1.25/1.0 = 1.25mol 2. Calculate ratio by dividing through by smallest number C:H = 0. ...
... A hydrocarbon contains 5.00g carbon and 1.25g hydrogen. One mole of the hydrocarbon has a mass of 30.00g 1. Calculate amount of each element amount (mol) = mass/molar mass amount C = 5.00/12.0 = 0.417mol amount H = 1.25/1.0 = 1.25mol 2. Calculate ratio by dividing through by smallest number C:H = 0. ...
chemistry advanced may 2010 marking scheme
... hydroxide ion are added by reacting with these species. (1) Chemical equations should be given to represent these changes. (1) (ii) Let the volume of 10-3M benzoate (A-) solution added to 100 cm3 of benzoic acid be V. Since the pH of mixture is 5.5 we can write: Ka = [H+][A-]/[HA] = 10-5.5 (10-3)[V/ ...
... hydroxide ion are added by reacting with these species. (1) Chemical equations should be given to represent these changes. (1) (ii) Let the volume of 10-3M benzoate (A-) solution added to 100 cm3 of benzoic acid be V. Since the pH of mixture is 5.5 we can write: Ka = [H+][A-]/[HA] = 10-5.5 (10-3)[V/ ...
8 SHS Ch 8 Lecture shs_ch_8_lecture_2012
... Example balancing chemical equations Step 1 . Insert 1 in front of the most complicated looking chemical ...
... Example balancing chemical equations Step 1 . Insert 1 in front of the most complicated looking chemical ...
Chapter 4 Student Notes
... This tends to stabilize the ions in solution and prevent cations and anions from recombining. The positive ions have the oxygen atoms of water pointing towards the ion; negative ions have the hydrogen atoms of water pointing towards the ion. The transport of ions through the solution causes electric ...
... This tends to stabilize the ions in solution and prevent cations and anions from recombining. The positive ions have the oxygen atoms of water pointing towards the ion; negative ions have the hydrogen atoms of water pointing towards the ion. The transport of ions through the solution causes electric ...
Chemistry 20
... excess) amount of sodium chloride combine to make a white precipitate silver chloride and some dissolved sodium nitrate. (i) How many moles of silver chloride are made? (ii) How many grams of silver chloride is that? (iii) How many moles of sodium nitrate are made? ...
... excess) amount of sodium chloride combine to make a white precipitate silver chloride and some dissolved sodium nitrate. (i) How many moles of silver chloride are made? (ii) How many grams of silver chloride is that? (iii) How many moles of sodium nitrate are made? ...
Chemistry (B) Final Exam Study Guide 3
... ____ 54. Which of the following statements is NOT true about what happens in all chemical reactions? a. The ways in which atoms are joined together are changed. b. New atoms are formed as products. c. The starting substances are called reactants. d. The bonds of the reactants are broken and new bon ...
... ____ 54. Which of the following statements is NOT true about what happens in all chemical reactions? a. The ways in which atoms are joined together are changed. b. New atoms are formed as products. c. The starting substances are called reactants. d. The bonds of the reactants are broken and new bon ...
Oxidation-Reduction Reactions
... reduction (electron gain). Collectively, oxidation and reduction are known as redox, or an electron transfer reaction. After balancing the two halfequations one can determine the total net reaction. Each equation is balanced by adjusting coefficients and adding H2O, H+, and e- in this order: 1) Bala ...
... reduction (electron gain). Collectively, oxidation and reduction are known as redox, or an electron transfer reaction. After balancing the two halfequations one can determine the total net reaction. Each equation is balanced by adjusting coefficients and adding H2O, H+, and e- in this order: 1) Bala ...
03_Worked_Examples
... Plan Because the molar mass of any substance is numerically equal to its formula weight, we first determine the formula weight of glucose by adding the atomic weights of its component atoms. The formula weight will have units of amu, whereas the molar mass has units of g/mol. Solve Our first step is ...
... Plan Because the molar mass of any substance is numerically equal to its formula weight, we first determine the formula weight of glucose by adding the atomic weights of its component atoms. The formula weight will have units of amu, whereas the molar mass has units of g/mol. Solve Our first step is ...
Chemical Equilibrium - Department of Chemistry
... and liquid phase. The vapor pressure of H2O at a given temperature is a property associated with an equilibrium condition. H2O(g) H2O(l) I2 originally dissolved in water (left) will partition between the CCl4 and H2O liquids such that [I2]CCl4/ [I2]H2O = 86. The distribution coefficient of a solute ...
... and liquid phase. The vapor pressure of H2O at a given temperature is a property associated with an equilibrium condition. H2O(g) H2O(l) I2 originally dissolved in water (left) will partition between the CCl4 and H2O liquids such that [I2]CCl4/ [I2]H2O = 86. The distribution coefficient of a solute ...
03_Worked_Examples
... Plan Because the molar mass of any substance is numerically equal to its formula weight, we first determine the formula weight of glucose by adding the atomic weights of its component atoms. The formula weight will have units of amu, whereas the molar mass has units of g/mol. Solve Our first step is ...
... Plan Because the molar mass of any substance is numerically equal to its formula weight, we first determine the formula weight of glucose by adding the atomic weights of its component atoms. The formula weight will have units of amu, whereas the molar mass has units of g/mol. Solve Our first step is ...
chm 434f/1206f solid state materials chemistry
... REACTIVITY OF SOLIDS - SUPERFICIALLY SIMPLE, INTRINSICALLY COMPLEX • Classical exchange or metathesis reactions • Look very simple, in practice actually extremely complicated • Consider zinc blende type reagents with dominant cation mobility ...
... REACTIVITY OF SOLIDS - SUPERFICIALLY SIMPLE, INTRINSICALLY COMPLEX • Classical exchange or metathesis reactions • Look very simple, in practice actually extremely complicated • Consider zinc blende type reagents with dominant cation mobility ...
H 2 SO 4
... an ionic compound made up of a cation other that H+ and an anion other that OH- or O2-: acid + base g salt + water HCl(aq) + NaOH(aq) g NaCl(aq) + H2O(l) All salts are strong electrolytes. The substance we know as table salt, NaCl, is a familiar example. However, since both the acid and the base are ...
... an ionic compound made up of a cation other that H+ and an anion other that OH- or O2-: acid + base g salt + water HCl(aq) + NaOH(aq) g NaCl(aq) + H2O(l) All salts are strong electrolytes. The substance we know as table salt, NaCl, is a familiar example. However, since both the acid and the base are ...
O 2 (g) - Valdosta State University
... Summary: Matter and Energy Dispersal • A final state of a system can be more probable than the initial state if: – The atoms and molecules can be more ____________ and/or – ___________ can be dispersed over a greater number of atoms and molecules. ...
... Summary: Matter and Energy Dispersal • A final state of a system can be more probable than the initial state if: – The atoms and molecules can be more ____________ and/or – ___________ can be dispersed over a greater number of atoms and molecules. ...
Introductory Chemistry I
... 1. 10 mL of 0.2 M NaOH solution is placed into a small beaker with a small amount of phenolphthalein. . If 0.1 M HCl is added dropwise: a. the solution will become red on the first mL added, then change to colorless after 5 mL have been added. b. the solution will become red after 20 mL have been ad ...
... 1. 10 mL of 0.2 M NaOH solution is placed into a small beaker with a small amount of phenolphthalein. . If 0.1 M HCl is added dropwise: a. the solution will become red on the first mL added, then change to colorless after 5 mL have been added. b. the solution will become red after 20 mL have been ad ...
Chemistry - Beachwood City Schools
... Answers to Chapter 11 Study Questions 1. Wavelength is inversely proportional to frequency. Energy is proportional to frequency. 2. The new idea in Bohr's model was that electrons can only exist in specific energy states. Bohr's model included an electron orbiting the nucleus as a planet does the su ...
... Answers to Chapter 11 Study Questions 1. Wavelength is inversely proportional to frequency. Energy is proportional to frequency. 2. The new idea in Bohr's model was that electrons can only exist in specific energy states. Bohr's model included an electron orbiting the nucleus as a planet does the su ...
Chemistry 12 is an intensive course, covering a great deal of
... A5 apply collision theory to explain how reaction rates can be changed use collision theory to explain the effect of the following factors on reaction rate: – nature of reactants – concentration – temperature – surface area A6 analyse the reaction mechanism for a reacting system 1. explain why most ...
... A5 apply collision theory to explain how reaction rates can be changed use collision theory to explain the effect of the following factors on reaction rate: – nature of reactants – concentration – temperature – surface area A6 analyse the reaction mechanism for a reacting system 1. explain why most ...
Chapter One
... analysis showed that glucose contains only carbon, hydrogen, and oxygen. Determine the empirical formula of the compound. ...
... analysis showed that glucose contains only carbon, hydrogen, and oxygen. Determine the empirical formula of the compound. ...
Section 6.3 Balancing Chemical Equations
... 1. Write the reactants as they actually exist before any reaction occurs. Remember that when a salt dissolves, its ions separate. 2. Consider the various solids that could form. To do this, simply exchange the anions of the added salts. 3. Use the solubility rules to decide whether a solid forms and ...
... 1. Write the reactants as they actually exist before any reaction occurs. Remember that when a salt dissolves, its ions separate. 2. Consider the various solids that could form. To do this, simply exchange the anions of the added salts. 3. Use the solubility rules to decide whether a solid forms and ...
Stoichiometry

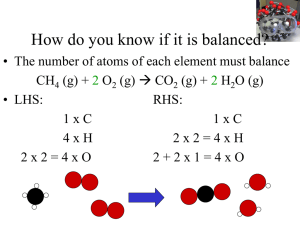
Stoichiometry /ˌstɔɪkiˈɒmɨtri/ is the calculation of relative quantities of reactants and products in chemical reactions.Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of product can be empirically determined, then the amount of the other reactants can also be calculated.As seen in the image to the right, where the balanced equation is:CH4 + 2 O2 → CO2 + 2 H2O.Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. Stoichiometry measures these quantitative relationships, and is used to determine the amount of products/reactants that are produced/needed in a given reaction. Describing the quantitative relationships among substances as they participate in chemical reactions is known as reaction stoichiometry. In the example above, reaction stoichiometry measures the relationship between the methane and oxygen as they react to form carbon dioxide and water.Because of the well known relationship of moles to atomic weights, the ratios that are arrived at by stoichiometry can be used to determine quantities by weight in a reaction described by a balanced equation. This is called composition stoichiometry.Gas stoichiometry deals with reactions involving gases, where the gases are at a known temperature, pressure, and volume and can be assumed to be ideal gases. For gases, the volume ratio is ideally the same by the ideal gas law, but the mass ratio of a single reaction has to be calculated from the molecular masses of the reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the mass ratio.