Teacher Key - 3D Molecular Designs
... not be able to bind the substrate and the reaction would slow down. ...
... not be able to bind the substrate and the reaction would slow down. ...
NATIONAL UNIVERSITY OF SINGAPORE DEPARTMENT OF BIOCHEMISTRY ADVANCED PLACEMENT TEST (SAMPLE)
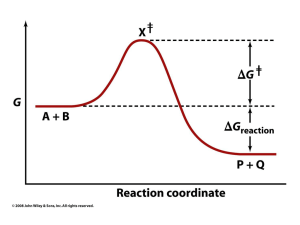
... 18. How do catalysts work to accelerate a chemical reaction? A. They raise the average free energy of the products. B. They provide a means of acceleration by being completely consumed in the reaction. C. They lower the energy of activation. D. They lower the overall free energy change of the react ...
... 18. How do catalysts work to accelerate a chemical reaction? A. They raise the average free energy of the products. B. They provide a means of acceleration by being completely consumed in the reaction. C. They lower the energy of activation. D. They lower the overall free energy change of the react ...
Food Safety & Toxicology (3) - Share My Knowledge & Experience
... • Source: mushroom • Reduction: Immediate blanching after cleaning and cutting can reduce the substance • Mechanism: condensation of the hydrazines with the carbonyl compounds pyridoxal and pyridoxal phosphate — the active form of the vitamin — resulting in the formation of inactive hydrazones ...
... • Source: mushroom • Reduction: Immediate blanching after cleaning and cutting can reduce the substance • Mechanism: condensation of the hydrazines with the carbonyl compounds pyridoxal and pyridoxal phosphate — the active form of the vitamin — resulting in the formation of inactive hydrazones ...
Protein Structure and Function
... If the transition state can be bound more tightly than the substrate, activation energy will be reduced The differential binding of enzyme for these two state Is the driving force of reactions ...
... If the transition state can be bound more tightly than the substrate, activation energy will be reduced The differential binding of enzyme for these two state Is the driving force of reactions ...
Slide 1
... 5. Three dimensional dcavity bearing a specific charge by which the enzyme reacts with its substrate is called • A.Active site B.Binding site 6. Which step causes activation of catalytic site of an enzyme? • A.Change in pH of the surroundings. • B.Formation of Enzyme Susstrate complex ...
... 5. Three dimensional dcavity bearing a specific charge by which the enzyme reacts with its substrate is called • A.Active site B.Binding site 6. Which step causes activation of catalytic site of an enzyme? • A.Change in pH of the surroundings. • B.Formation of Enzyme Susstrate complex ...
Principles of immunological Techniques
... measurement s are taken at time intervals to determine the speed of the enzyme reaction. ...
... measurement s are taken at time intervals to determine the speed of the enzyme reaction. ...
lec-08-handout
... After talking about reversible enzyme inhibition, now let’s discuss irreversible enzyme inhibition. The inhibitor is very tightly bound to enzyme either covalently or non-covalently in such a way that this inhibition is irreversible. Let’s take the very classical example penicillin, the first antibi ...
... After talking about reversible enzyme inhibition, now let’s discuss irreversible enzyme inhibition. The inhibitor is very tightly bound to enzyme either covalently or non-covalently in such a way that this inhibition is irreversible. Let’s take the very classical example penicillin, the first antibi ...
Lecture 9: Biological Pathway Simulation
... Rate Constants and Reaction Orders • Each reaction is characterized by its own rate constant, depending on the nature of the reactants and the temperature • In general, the order with respect to each ...
... Rate Constants and Reaction Orders • Each reaction is characterized by its own rate constant, depending on the nature of the reactants and the temperature • In general, the order with respect to each ...
A3.3.1ActionMolecules
... have a variety of shapes, sizes, chemical compositions, and chemical reactivity. They include thousands of different substances which can be classified into five basic types: structural, regulatory, immunological, transport and catalytic. In this activity, you will focus on the action or catalytic p ...
... have a variety of shapes, sizes, chemical compositions, and chemical reactivity. They include thousands of different substances which can be classified into five basic types: structural, regulatory, immunological, transport and catalytic. In this activity, you will focus on the action or catalytic p ...
12.1 Mechanisms regulating enzyme synthesis 12.1.2.2 Enzyme
... Microbial ecosystems are oligotrophic with a limited availability of nutrients. Furthermore, nutrients are not usually found in balanced concentrations while the organisms have to compete with each other for available nutrients. Organic materials are converted to carbon skeletons for monomer a ...
... Microbial ecosystems are oligotrophic with a limited availability of nutrients. Furthermore, nutrients are not usually found in balanced concentrations while the organisms have to compete with each other for available nutrients. Organic materials are converted to carbon skeletons for monomer a ...
What is an enzyme? Func
... • When a substrate (S) fits properly in an ac9ve site, an enzyme-‐substrate (ES) complex is formed: E + S D ES • Within the ac9ve site of the ES complex, the reac9on occurs ...
... • When a substrate (S) fits properly in an ac9ve site, an enzyme-‐substrate (ES) complex is formed: E + S D ES • Within the ac9ve site of the ES complex, the reac9on occurs ...
Document
... Using recombinant DNA procedures, it is possible to modify a gene to use a different amino acid in a protein sequence. • Assists in the study of enzyme structure and activity. • Allow for the design of new enzymes and other proteins with desired properties. • The approach can be used for the design ...
... Using recombinant DNA procedures, it is possible to modify a gene to use a different amino acid in a protein sequence. • Assists in the study of enzyme structure and activity. • Allow for the design of new enzymes and other proteins with desired properties. • The approach can be used for the design ...
C) the gain of electrons.
... interfere with the cell's abilities to catalyze various reactions. C) Elevated body temperatures will increase the energy of activation needed to start various chemical reactions in the body. This will interfere with the ability of enzymes to catalyze vital chemical reactions. D) Elevated body tempe ...
... interfere with the cell's abilities to catalyze various reactions. C) Elevated body temperatures will increase the energy of activation needed to start various chemical reactions in the body. This will interfere with the ability of enzymes to catalyze vital chemical reactions. D) Elevated body tempe ...
CHEMICAL REACTIONS, ENZYMES, ATP, CELLULAR
... 2. What are the 3 phases of the cellular respiration process in order? 3. Where in the cell does the glycolysis part of cellular respiration occur? 4. Is glycolysis aerobic or anaerobic? 5. Where in the ...
... 2. What are the 3 phases of the cellular respiration process in order? 3. Where in the cell does the glycolysis part of cellular respiration occur? 4. Is glycolysis aerobic or anaerobic? 5. Where in the ...
irm_ch21
... 21.24 apoenzyme + cofactor = holoenzyme = conjugated enzyme 21.25 An enzyme active site is the relatively small part of an enzyme that is actually involved in the process of catalysis. 21.26 an intermediate species that is formed when a substrate binds to the active site of an enzyme 21.27 In the lo ...
... 21.24 apoenzyme + cofactor = holoenzyme = conjugated enzyme 21.25 An enzyme active site is the relatively small part of an enzyme that is actually involved in the process of catalysis. 21.26 an intermediate species that is formed when a substrate binds to the active site of an enzyme 21.27 In the lo ...
Introduction to Enzymes - Worthington Biochemical
... The living cell is the site of tremendous biochemical activity called metabolism. This is the process of chemical and physical change which goes on continually in the living organism. Build-up of new tissue, replacement of old tissue, conversion of food to energy, disposal of waste materials, reprod ...
... The living cell is the site of tremendous biochemical activity called metabolism. This is the process of chemical and physical change which goes on continually in the living organism. Build-up of new tissue, replacement of old tissue, conversion of food to energy, disposal of waste materials, reprod ...
EXAM I (September 21, 2005) BIOCHEMISTRY 460 9:00 am section
... 7. Remembering that )G = )G0' + 1.36 log [products]/[substrates], hydrolysis reactions have a large negative )G0'. In general would you expect these reactions to be spontaneous (yes or no), and in general terms (use words not math) under what conditions would you expect the reaction not to be sponta ...
... 7. Remembering that )G = )G0' + 1.36 log [products]/[substrates], hydrolysis reactions have a large negative )G0'. In general would you expect these reactions to be spontaneous (yes or no), and in general terms (use words not math) under what conditions would you expect the reaction not to be sponta ...
ATP
... via either the de novo or salvage pathways • For the de novo pathway, amino acids (Asp, Gln, Gly), ribose 5-phosphate, CO2, One-carbon units (carried by H4 folate) are the precursors. • The free bases are not intermediates for the de ...
... via either the de novo or salvage pathways • For the de novo pathway, amino acids (Asp, Gln, Gly), ribose 5-phosphate, CO2, One-carbon units (carried by H4 folate) are the precursors. • The free bases are not intermediates for the de ...
19 Dr. Nafez Abu Tarboosh Qusai Al Sharef
... carbonyl ketone group (on C 2) so the bonds around this carbon will be weaken (between c1 and c2) and C1 will leave as a carboxylic group and this is why we call it decarboxylation reaction. Thiamine is rapidly converted to its active form thiamine pyrophosphate (TPP) in the brain and liver. ...
... carbonyl ketone group (on C 2) so the bonds around this carbon will be weaken (between c1 and c2) and C1 will leave as a carboxylic group and this is why we call it decarboxylation reaction. Thiamine is rapidly converted to its active form thiamine pyrophosphate (TPP) in the brain and liver. ...
Enzyme - Wesleyan College Faculty
... 3 Active site (and R groups of its amino acids) can lower EA and speed up a reaction by • acting as a template for substrate orientation, • stressing the substrates and stabilizing the ...
... 3 Active site (and R groups of its amino acids) can lower EA and speed up a reaction by • acting as a template for substrate orientation, • stressing the substrates and stabilizing the ...
Document
... Non-availability of the receptor structure is a bottleneck… In our pursuit to engage with experimentalists for lead discovery or optimization, our efforts become restricted in the absence of an experimental structure of the receptor protein/enzyme. When we analyze, it occurred to us that most of th ...
... Non-availability of the receptor structure is a bottleneck… In our pursuit to engage with experimentalists for lead discovery or optimization, our efforts become restricted in the absence of an experimental structure of the receptor protein/enzyme. When we analyze, it occurred to us that most of th ...
UNIT 2 BIOLOGICAL CHEMISTRY ORGANIC MOLECULES
... Co-factors: Inorganic ions that bind to the enzyme to help catalyze the reaction Co-enzymes: Organic molecules (not proteins) -generally are bound at the active site and the enzymatic rxn will not work w/o it. Coenzymes are found in small con. Because they are ...
... Co-factors: Inorganic ions that bind to the enzyme to help catalyze the reaction Co-enzymes: Organic molecules (not proteins) -generally are bound at the active site and the enzymatic rxn will not work w/o it. Coenzymes are found in small con. Because they are ...
Proteins
... causing it to uncoil or form a new shape. This is caused by heat, pH, or radiation. This change is not permanent Coagulation is a permanent change in the structure. Example is a boiled egg ...
... causing it to uncoil or form a new shape. This is caused by heat, pH, or radiation. This change is not permanent Coagulation is a permanent change in the structure. Example is a boiled egg ...
Enzyme inhibitor

An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both.Many drug molecules are enzyme inhibitors, so their discovery and improvement is an active area of research in biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, like proteases or nucleases. A well-characterised example of this is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions. Natural enzyme inhibitors can also be poisons and are used as defences against predators or as ways of killing prey.