Chapter 8
... enzyme, competing with the substrate • Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective • Examples of inhibitors include toxins, poisons, pesticides, and antibiotics ...
... enzyme, competing with the substrate • Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective • Examples of inhibitors include toxins, poisons, pesticides, and antibiotics ...
Assay of Enzymes with Insoluble or Unknown - Beilstein
... will have the same EC class, unless it can be demonstrated that they have different substrate specificity. For example in aerobic conditions the enzyme that oxidizes succinate to fumarate, as in mitochondria, is Complex II, succinate: ubiquinone reductase [16]. Under anaerobic conditions expression ...
... will have the same EC class, unless it can be demonstrated that they have different substrate specificity. For example in aerobic conditions the enzyme that oxidizes succinate to fumarate, as in mitochondria, is Complex II, succinate: ubiquinone reductase [16]. Under anaerobic conditions expression ...
Today`s Plan: 1/5/09
... High temperature, extreme salinity and pH changes can cause denaturating of proteins=protein becomes misshapen because the forces controlling the levels of structure above have been ...
... High temperature, extreme salinity and pH changes can cause denaturating of proteins=protein becomes misshapen because the forces controlling the levels of structure above have been ...
allosteric activator
... Cellular enzyme proteins are in a dynamic state of turn over, with the relative rates of enzyme synthesis and degradation ultimately determining the amount of enzymes. In many instances, transcriptional regulation determines the concentrations of specific enzyme, with enzyme proteins degradation pla ...
... Cellular enzyme proteins are in a dynamic state of turn over, with the relative rates of enzyme synthesis and degradation ultimately determining the amount of enzymes. In many instances, transcriptional regulation determines the concentrations of specific enzyme, with enzyme proteins degradation pla ...
Enzymes - part 1
... Catalysts - change rate of reaction without net change of catalyst Catalyst does not alter equilibrium ...
... Catalysts - change rate of reaction without net change of catalyst Catalyst does not alter equilibrium ...
Chapter Nineteen
... ► In reversible inhibition, the inhibitor can leave, restoring the enzyme to its uninhibited level of activity. ► In irreversible inhibition, the inhibitor remains permanently bound and the enzyme is permanently inhibited. ► The inhibition can also be competitive or noncompetitive, depending on whet ...
... ► In reversible inhibition, the inhibitor can leave, restoring the enzyme to its uninhibited level of activity. ► In irreversible inhibition, the inhibitor remains permanently bound and the enzyme is permanently inhibited. ► The inhibition can also be competitive or noncompetitive, depending on whet ...
BIOCHEMISTRY NOTES
... 1. These are molecules that sometimes have to be present in order for an enzyme to perform its function 2. Some enzymes only become active when all the appropriate cofactors or coenzymes are present and bind to the appropriate sites on the enzyme F. ALLOSTERIC INTERACTIONS 1. An allosteric interacti ...
... 1. These are molecules that sometimes have to be present in order for an enzyme to perform its function 2. Some enzymes only become active when all the appropriate cofactors or coenzymes are present and bind to the appropriate sites on the enzyme F. ALLOSTERIC INTERACTIONS 1. An allosteric interacti ...
Molecular modeling of HIV-1 reverse
... resistance to NNIRTs, it was felt that the method was valid within these limits. This model was modified separately at each of 12 key amino acid residues (see Table I) and the resultant sites were subjected to molecular mechanics energy minimization. Nine of the amino acid residues that were modifie ...
... resistance to NNIRTs, it was felt that the method was valid within these limits. This model was modified separately at each of 12 key amino acid residues (see Table I) and the resultant sites were subjected to molecular mechanics energy minimization. Nine of the amino acid residues that were modifie ...
9.6 Respiration 4 (Control and other metabolites)
... – no unnecessary accumulation of product – production is self-limiting ...
... – no unnecessary accumulation of product – production is self-limiting ...
Practice Exam III
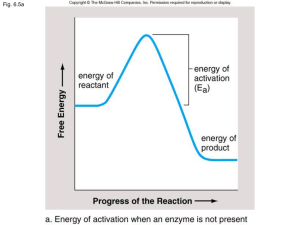
... a). Enzymes theoretically stabilize the transition state of the reaction they catalyze. b). Enzymes alter the equilibrium distribution of the substrate(s) and product(s) of the reaction they catalyze. c). Enzymes reduce the activation energy of the reaction they catalyze. d). Enzymes employ a wide v ...
... a). Enzymes theoretically stabilize the transition state of the reaction they catalyze. b). Enzymes alter the equilibrium distribution of the substrate(s) and product(s) of the reaction they catalyze. c). Enzymes reduce the activation energy of the reaction they catalyze. d). Enzymes employ a wide v ...
Design of Tight-Binding Human Immunodeficiency
... treatment of chronic disease, high oral bioavailability must be accompanied by long elimination half-life to yield sustained virus-suppressive drug level in the blood and infected tissue. This requirement is prescribed by the profound ability of HIV to develop resistance under selective antiviral pr ...
... treatment of chronic disease, high oral bioavailability must be accompanied by long elimination half-life to yield sustained virus-suppressive drug level in the blood and infected tissue. This requirement is prescribed by the profound ability of HIV to develop resistance under selective antiviral pr ...
5_Bio_1_ReKaps
... Induced Fit: the presence of the appropriate substrate causes a conformational change in the enzyme such that the enzyme and substrate fit together (the accepted theory) Reaction Energy Profiles: an enzyme changes the rate of a reaction, but not its equilibrium point or whether it will be spontane ...
... Induced Fit: the presence of the appropriate substrate causes a conformational change in the enzyme such that the enzyme and substrate fit together (the accepted theory) Reaction Energy Profiles: an enzyme changes the rate of a reaction, but not its equilibrium point or whether it will be spontane ...
The Organic Chemistry of Enzyme Catalyzed Reactions Revised
... Hypothesized that an enzyme is a flexible template that is most complementary to substrates at the transition state rather than at the ground state Therefore, the substrate does not bind most effectively in the ES complex As reaction proceeds, enzyme conforms better to the transition-state structur ...
... Hypothesized that an enzyme is a flexible template that is most complementary to substrates at the transition state rather than at the ground state Therefore, the substrate does not bind most effectively in the ES complex As reaction proceeds, enzyme conforms better to the transition-state structur ...
powerpoint
... Ribose sugar component may be converted to ribose-5-phosphate which is a substrate for PRPP Synthetase Ribose sugar component may be further catabolized in HMP pathway ...
... Ribose sugar component may be converted to ribose-5-phosphate which is a substrate for PRPP Synthetase Ribose sugar component may be further catabolized in HMP pathway ...
TheraGest - ProThera
... The enzymes contained in this formulation are provided by pancreatin, a potent extract from porcine pancreas that contains enzymes specific for digestion of fat, protein, and starch. The USP pancreatin material contained in this product provides the following enzyme activity: • Lipase (Fat-specific) ...
... The enzymes contained in this formulation are provided by pancreatin, a potent extract from porcine pancreas that contains enzymes specific for digestion of fat, protein, and starch. The USP pancreatin material contained in this product provides the following enzyme activity: • Lipase (Fat-specific) ...
Lecture Slides for Fatty Acid Catabolism
... • daily requirement is about 2-3 mg/day • Gastric mucosa produces a protein called intrinsic factor • Lack of intrinsic factor results in impaired B12 absorption, pernicious anemia, death in 1-3 years • Original treatment (1920’s) was ½ lb. of raw liver daily • Concentrated liver juice (yum) became ...
... • daily requirement is about 2-3 mg/day • Gastric mucosa produces a protein called intrinsic factor • Lack of intrinsic factor results in impaired B12 absorption, pernicious anemia, death in 1-3 years • Original treatment (1920’s) was ½ lb. of raw liver daily • Concentrated liver juice (yum) became ...
Enzyme
... When the active site is prevented from combining with the substrate, INHIBITION occurs This can be a very effective way of controlling reaction rates. In some reactions, the PRODUCT competes with the substrate. High Product means lower reaction rate! In a Metabolic Pathway, The end product may be su ...
... When the active site is prevented from combining with the substrate, INHIBITION occurs This can be a very effective way of controlling reaction rates. In some reactions, the PRODUCT competes with the substrate. High Product means lower reaction rate! In a Metabolic Pathway, The end product may be su ...
Revision PPT on enzymes File
... The shape of an enzyme is very important because it has a direct effect on how it catalyzes a reaction. Why do enzymes have different shapes? An enzyme’s shape is determined by the sequence of amino acids in its structure, and the bonds which form between the atoms of those molecules. ...
... The shape of an enzyme is very important because it has a direct effect on how it catalyzes a reaction. Why do enzymes have different shapes? An enzyme’s shape is determined by the sequence of amino acids in its structure, and the bonds which form between the atoms of those molecules. ...
Middle-Term Test Paper on Biochemistry
... Isoenzymes ( isozymes ) are different forms of an enzyme which catalyze the same reaction, but exhibit different physical or kinetic properties, such as isoelectric point, pH optimum, substrate affinity or effect of inhibitors. 3) zymogen and its activation Several enzymes are synthesized as larger ...
... Isoenzymes ( isozymes ) are different forms of an enzyme which catalyze the same reaction, but exhibit different physical or kinetic properties, such as isoelectric point, pH optimum, substrate affinity or effect of inhibitors. 3) zymogen and its activation Several enzymes are synthesized as larger ...
amino acids
... The shape of an enzyme is very important because it has a direct effect on how it catalyzes a reaction. Why do enzymes have different shapes? An enzyme’s shape is determined by the sequence of amino acids in its structure, and the bonds which form between the atoms of those molecules. ...
... The shape of an enzyme is very important because it has a direct effect on how it catalyzes a reaction. Why do enzymes have different shapes? An enzyme’s shape is determined by the sequence of amino acids in its structure, and the bonds which form between the atoms of those molecules. ...
Chemistry, Biomolecules, and Enzymes
... Substrate = reactant an enzyme acts on, converts substrate to product Enzyme-Substrate Complex = forms when enzyme and substrate join Enzyme will only act on its specific substrate – dependent on shape Active Site = region of enzyme that substrate binds to Induced fit = enzyme changes shape to fit m ...
... Substrate = reactant an enzyme acts on, converts substrate to product Enzyme-Substrate Complex = forms when enzyme and substrate join Enzyme will only act on its specific substrate – dependent on shape Active Site = region of enzyme that substrate binds to Induced fit = enzyme changes shape to fit m ...
Partial Class Notes Chapter 6-8 ENZYME#2
... _______________: "weak" binding ( ~0.1 M) of S to active site raises the effective concentration of S and favors more frequent transition states 104-105 effective molarity: enhanced relative concentration of reactants due to binding to E ____________________: greater binding of transition states ...
... _______________: "weak" binding ( ~0.1 M) of S to active site raises the effective concentration of S and favors more frequent transition states 104-105 effective molarity: enhanced relative concentration of reactants due to binding to E ____________________: greater binding of transition states ...
Partial Class Notes Chapter 6-8 ENZYME#2
... the new N-terminal, producing an NH3+ that turns inward and interacts with the sidechain carboxyl of Asp194, forming an ion pair. This opens up the binding pocket. The three residues shown in red are the catalytic triad. ...
... the new N-terminal, producing an NH3+ that turns inward and interacts with the sidechain carboxyl of Asp194, forming an ion pair. This opens up the binding pocket. The three residues shown in red are the catalytic triad. ...
Cycle Krebs Worksheet - LTE - IB
... * The production of Citrate, α-‐ketoglutarate, and Succinyl-‐CoA involves irreversible reactions. * Decarboxylation of α-‐ketoglutarate is catalyzed by an enzymatic complex, similar to Piruvate Dehydrogenase ...
... * The production of Citrate, α-‐ketoglutarate, and Succinyl-‐CoA involves irreversible reactions. * Decarboxylation of α-‐ketoglutarate is catalyzed by an enzymatic complex, similar to Piruvate Dehydrogenase ...
Enzyme inhibitor

An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both.Many drug molecules are enzyme inhibitors, so their discovery and improvement is an active area of research in biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, like proteases or nucleases. A well-characterised example of this is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions. Natural enzyme inhibitors can also be poisons and are used as defences against predators or as ways of killing prey.