Document
... and catalyze the reaction? • What is the spatial relationship of the essential amino acids residues in the active site? • What is the mechanism by which the essential amino acid residues catalyze the reaction? • As a model, we consider chymotrypsin, an enzyme of the digestive system that catalyzes t ...
... and catalyze the reaction? • What is the spatial relationship of the essential amino acids residues in the active site? • What is the mechanism by which the essential amino acid residues catalyze the reaction? • As a model, we consider chymotrypsin, an enzyme of the digestive system that catalyzes t ...
end of semester main examination - UR-CST
... EDTA (ethylenediaminetetraacetic acid) results in the loss of enzyme activity. Provide an explanation. (2 marks) v. An enzyme that follows Michaelis–Menten kinetics has a Km of 1 μM. The initial velocity is 0.1 μM min-1 at a substrate concentration of 100 μM. Determine the initial velocity when [S] ...
... EDTA (ethylenediaminetetraacetic acid) results in the loss of enzyme activity. Provide an explanation. (2 marks) v. An enzyme that follows Michaelis–Menten kinetics has a Km of 1 μM. The initial velocity is 0.1 μM min-1 at a substrate concentration of 100 μM. Determine the initial velocity when [S] ...
ENZYME KINETICS - University of Pennsylvania
... The interaction of E and I does not interfere with the S binding to E, so that ESI may be formed in addition to EI. But ES is sensitive to the presence of I, rendering ESI catalytically inactive or less active. Since the interaction between E and S is not being interfered with, any E not bound by I ...
... The interaction of E and I does not interfere with the S binding to E, so that ESI may be formed in addition to EI. But ES is sensitive to the presence of I, rendering ESI catalytically inactive or less active. Since the interaction between E and S is not being interfered with, any E not bound by I ...
Biology - PHA Science
... a) Diagram an amino acid and label its functional groups. What is the importance of the R group? b) Describe the four different levels of protein structure. What kind of chemical interactions (e.g. hydrogen bonds, peptide bonds, covalent bonds, hydrophobic interactions) does each level depend on? c) ...
... a) Diagram an amino acid and label its functional groups. What is the importance of the R group? b) Describe the four different levels of protein structure. What kind of chemical interactions (e.g. hydrogen bonds, peptide bonds, covalent bonds, hydrophobic interactions) does each level depend on? c) ...
Enzymes II – How Enzymes Work
... the enzyme pepsin, found in your normally acidic stomach, functions best at a very acidic pH of around 2. Slide 6 As with pH, most enzymes also have a particular temperature at which they function best, called the optimal temperature. As a general rule, enzyme activity increases with increasing temp ...
... the enzyme pepsin, found in your normally acidic stomach, functions best at a very acidic pH of around 2. Slide 6 As with pH, most enzymes also have a particular temperature at which they function best, called the optimal temperature. As a general rule, enzyme activity increases with increasing temp ...
Notes - Organic Molecules of Life
... All are isomers of C12H22O11 Formed by dehydration synthesis (requires enzymes) Examples of Disaccharides: ______________ – table sugar (Glucose + Fructose) ______________ – seed sugar (Glucose + Glucose) _______________ – milk sugar (Glucose + Galactose) Polysaccharides - Large, complex chains of _ ...
... All are isomers of C12H22O11 Formed by dehydration synthesis (requires enzymes) Examples of Disaccharides: ______________ – table sugar (Glucose + Fructose) ______________ – seed sugar (Glucose + Glucose) _______________ – milk sugar (Glucose + Galactose) Polysaccharides - Large, complex chains of _ ...
Biochemical Pathways – Legends General Remarks for
... 31) Mitochondrial chain elongation of palmityl-CoA occurs by reversal of β-oxidation. Only enoyl-CoA reductase is different. The microsomal system employs malonyl-CoA instead of acetyl-CoA.. 32) The yeast system is shown here. The central SH group is written at the bottom, the marginal SH group at ...
... 31) Mitochondrial chain elongation of palmityl-CoA occurs by reversal of β-oxidation. Only enoyl-CoA reductase is different. The microsomal system employs malonyl-CoA instead of acetyl-CoA.. 32) The yeast system is shown here. The central SH group is written at the bottom, the marginal SH group at ...
Chemical Reactions and Enzymes
... and block the active site 2. Noncompetitive inhibitors: bind to the enzyme causing its shape to change, changing the active site. ...
... and block the active site 2. Noncompetitive inhibitors: bind to the enzyme causing its shape to change, changing the active site. ...
CHAPTER 5 Energy and Life.
... FERMENTATION: IS ANAEROBIC…it DOES NOT need oxygen. This is thought to be an ANCIENT way for a cell to operate and suggests that CELLS were at one point able to survive on an Earth with little of NO oxygen on it. This takes place in the CYTOPLASM of the cell. ...
... FERMENTATION: IS ANAEROBIC…it DOES NOT need oxygen. This is thought to be an ANCIENT way for a cell to operate and suggests that CELLS were at one point able to survive on an Earth with little of NO oxygen on it. This takes place in the CYTOPLASM of the cell. ...
ClickThisLinkForEntries
... step of the pathway. Allosteric inhibitors ensure that there is the right amount of reactants and products to ensure maximum pathway efficiency. A picture from the textbook on the right shows how they work. Interactions of enzymes with allosteric inhibitors affect their structure; they change their ...
... step of the pathway. Allosteric inhibitors ensure that there is the right amount of reactants and products to ensure maximum pathway efficiency. A picture from the textbook on the right shows how they work. Interactions of enzymes with allosteric inhibitors affect their structure; they change their ...
Chapter 8 - Trimble County Schools
... • Competitive inhibitors bind to the active site of an enzyme, competing with the substrate • Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less ...
... • Competitive inhibitors bind to the active site of an enzyme, competing with the substrate • Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less ...
enzymes
... • The are usually specific and they work at low concentrations • They block the enzyme but they do not usually destroy it • Many drugs and poisons are inhibitors of enzymes in the nervous system ...
... • The are usually specific and they work at low concentrations • They block the enzyme but they do not usually destroy it • Many drugs and poisons are inhibitors of enzymes in the nervous system ...
Microbiology bio 123
... When the curve is based on temperature, the value is a curve that has the highest point at 35 degrees c. It begins at 0 degrees and ends at 70 degrees c. The colder the temperature, the slower the molecules move, so the speed of the enzyme slows when it get too cold but still functions. At about 69 ...
... When the curve is based on temperature, the value is a curve that has the highest point at 35 degrees c. It begins at 0 degrees and ends at 70 degrees c. The colder the temperature, the slower the molecules move, so the speed of the enzyme slows when it get too cold but still functions. At about 69 ...
Pancreatic Enzyme Formula
... Percentage of ingredient released in simulated gastric fluids and intestinal fluids.† After two hours in pH 1.2 simlated gastric fluids, less than 20% of capsule contents were released. After 45 minutes in pH 6.8 simulated intestinal fluids, more than 80% of capsule contents had dissolved. This test ...
... Percentage of ingredient released in simulated gastric fluids and intestinal fluids.† After two hours in pH 1.2 simlated gastric fluids, less than 20% of capsule contents were released. After 45 minutes in pH 6.8 simulated intestinal fluids, more than 80% of capsule contents had dissolved. This test ...
CH 2 -CH 2 -CH 2 -CH 2 -CH 2
... which speeds up the rate of a chemical reaction without entering the reaction itself • enzymes: organic catalysts made of protein • most enzyme names end in -ase • enzymes lower the energy needed to start a ...
... which speeds up the rate of a chemical reaction without entering the reaction itself • enzymes: organic catalysts made of protein • most enzyme names end in -ase • enzymes lower the energy needed to start a ...
metabolism 8.1 worksheet
... Outline the effect of increasing the substrate concentration on the control reaction (no inhibition). ...
... Outline the effect of increasing the substrate concentration on the control reaction (no inhibition). ...
Enzymes
... • This enzyme has an active site for fructose-6-phosphate molecules to bind with another phosphate group • It has an allosteric site for ATP molecules, the inhibitor • When the cell consumes a lot of ATP the level of ATP in the cell falls • No ATP binds to the allosteric site of phosphofructokinase ...
... • This enzyme has an active site for fructose-6-phosphate molecules to bind with another phosphate group • It has an allosteric site for ATP molecules, the inhibitor • When the cell consumes a lot of ATP the level of ATP in the cell falls • No ATP binds to the allosteric site of phosphofructokinase ...
Life Science Name: Date: ______ Per: ______ Chemical Reactions
... Chemical Reactions and Enzymes Standard: 1.b.Students know enzymes are proteins that catalyze biochemical reactions without altering the reaction equilibrium and the activities of enzymes depend on the temperature, ionic conditions, and the pH of the surroundings. Chemical Reactions (use pages 50-53 ...
... Chemical Reactions and Enzymes Standard: 1.b.Students know enzymes are proteins that catalyze biochemical reactions without altering the reaction equilibrium and the activities of enzymes depend on the temperature, ionic conditions, and the pH of the surroundings. Chemical Reactions (use pages 50-53 ...
1 Enzyme Mechanisms Topics: TIM, Chymotrypsin, Rate
... expected from the V vs pH bell curve discussed above. A closer look at the enzymesubstrate complex shows that this discrepancy might be explained by the proximity of His to nearby atoms. It was shown that replacing Glu with its shorter counterpart Asp, thus changing the distance between the carbonyl ...
... expected from the V vs pH bell curve discussed above. A closer look at the enzymesubstrate complex shows that this discrepancy might be explained by the proximity of His to nearby atoms. It was shown that replacing Glu with its shorter counterpart Asp, thus changing the distance between the carbonyl ...
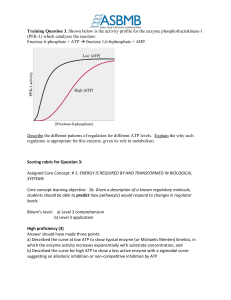
Training Question 3: Rubric
... 1. Answered provided only two of the a, b and c above OR 2. Answered stated two of a, b and c above correctly, but stated the third of these incorrectly OR 3. Three of the above except that for c) stated enzyme was inhibited at high ATP, but did not mention sigmoidal/allosteric pattern OR 4. Three o ...
... 1. Answered provided only two of the a, b and c above OR 2. Answered stated two of a, b and c above correctly, but stated the third of these incorrectly OR 3. Three of the above except that for c) stated enzyme was inhibited at high ATP, but did not mention sigmoidal/allosteric pattern OR 4. Three o ...
Lecture 19 - University of Wisconsin–Madison
... to provide adequate coordination of the metal and to place the catalytic bases on opposite sides of the substrate. There is no strict structural requirement that the metal ligands lie on the third, fourth, and fifth strands. ...
... to provide adequate coordination of the metal and to place the catalytic bases on opposite sides of the substrate. There is no strict structural requirement that the metal ligands lie on the third, fourth, and fifth strands. ...
Enzyme inhibitor

An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both.Many drug molecules are enzyme inhibitors, so their discovery and improvement is an active area of research in biochemistry and pharmacology. A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, like proteases or nucleases. A well-characterised example of this is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions. Natural enzyme inhibitors can also be poisons and are used as defences against predators or as ways of killing prey.