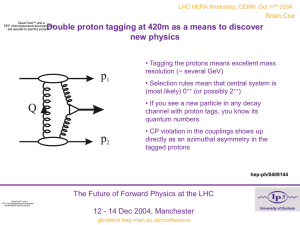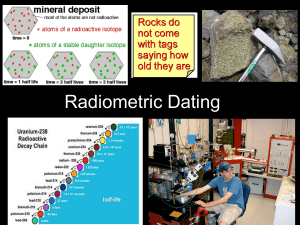
Core Problem - Max-Planck
... great stir – unlike the charge radius. Even the latter wouldn’t have been an issue if the new measurements had further narrowed the range of the charge radius as indicated by less accurate experiments. However, the fact that they put the radius in a different range altogether raises fundamental ques ...
... great stir – unlike the charge radius. Even the latter wouldn’t have been an issue if the new measurements had further narrowed the range of the charge radius as indicated by less accurate experiments. However, the fact that they put the radius in a different range altogether raises fundamental ques ...
Atomic structure and periodic table
... It can donate the three outer electrons to have stable electronic structure/configuration 2:8. It can gain five extra electrons to have stable electronic structure/configuration 2:8:8. Donating requires less energy, and thus Aluminium reacts by donating its three outer electrons. Elements with less ...
... It can donate the three outer electrons to have stable electronic structure/configuration 2:8. It can gain five extra electrons to have stable electronic structure/configuration 2:8:8. Donating requires less energy, and thus Aluminium reacts by donating its three outer electrons. Elements with less ...
03_DetectOverview
... The measured value was 104. Probably d was slightly less than its nominal value. It is good design practice to observe one or the other limit: A much smaller than the beam, in which case the IC can be regarded as sampling the local value of the dose, or much larger (‘integral chamber’) in which case ...
... The measured value was 104. Probably d was slightly less than its nominal value. It is good design practice to observe one or the other limit: A much smaller than the beam, in which case the IC can be regarded as sampling the local value of the dose, or much larger (‘integral chamber’) in which case ...
FlerasLectures - University of Oklahoma
... Modern particle physics began in the early 20th century as an exploration into the structure of the atom. The discovery of the atomic nucleus in the gold foil experiment of Geiger, Marsden, and Rutherford was the foundation of the field. The components of the nucleus were subsequently discovered in ...
... Modern particle physics began in the early 20th century as an exploration into the structure of the atom. The discovery of the atomic nucleus in the gold foil experiment of Geiger, Marsden, and Rutherford was the foundation of the field. The components of the nucleus were subsequently discovered in ...
Answer Key
... The neutral metal ball is attracted to the negatively charged bell. The excess electrons on the negatively charged bell are then transferred by conduction onto the metal ball causing the ball to become net negatively charged. The negatively charged ball is then repelled from the bell and collides wi ...
... The neutral metal ball is attracted to the negatively charged bell. The excess electrons on the negatively charged bell are then transferred by conduction onto the metal ball causing the ball to become net negatively charged. The negatively charged ball is then repelled from the bell and collides wi ...
Atoms and Molecules
... electrons by two atoms. • If two atoms come close enough that their unshared orbitals overlap, each atom can count both electrons toward its goal of filling the valence shell. • For example, if two hydrogen atoms come close enough that their 1s orbitals overlap, then they can share the single electr ...
... electrons by two atoms. • If two atoms come close enough that their unshared orbitals overlap, each atom can count both electrons toward its goal of filling the valence shell. • For example, if two hydrogen atoms come close enough that their 1s orbitals overlap, then they can share the single electr ...
Basics of Particle Physics - The University of Oklahoma
... Modern particle physics began in the early 20th century as an exploration into the structure of the atom. The discovery of the atomic nucleus in the gold foil experiment of Geiger, Marsden, and Rutherford was the foundation of the field. The components of the nucleus were subsequently discovered in ...
... Modern particle physics began in the early 20th century as an exploration into the structure of the atom. The discovery of the atomic nucleus in the gold foil experiment of Geiger, Marsden, and Rutherford was the foundation of the field. The components of the nucleus were subsequently discovered in ...
In terms of ANSWERS
... 5. In terms of the collision theory, state why adding extra N2 produces more NH3 more N2 molecules means more molecules present to potentially collide, thus producing more NH3 6. In terms of LeChatelier’s principle, state why adding extra N2 produces more NH3 adding N2 makes the equilibrium reaction ...
... 5. In terms of the collision theory, state why adding extra N2 produces more NH3 more N2 molecules means more molecules present to potentially collide, thus producing more NH3 6. In terms of LeChatelier’s principle, state why adding extra N2 produces more NH3 adding N2 makes the equilibrium reaction ...
www.XtremePapers.com
... 13 The centre of the Sun produces large amounts of energy. What is the source of this energy? A ...
... 13 The centre of the Sun produces large amounts of energy. What is the source of this energy? A ...
rtnedhistlnkedchp - Churinga Publishing Home Page
... thought experiment, but another answer lies in the electromagnetic field which is postulated in the previous block (Paper 10). This argument is pursued in the next paper by comparing the Doppler effect for sound and light. The conclusion is that the mechanisms are fundamentally different, and redshi ...
... thought experiment, but another answer lies in the electromagnetic field which is postulated in the previous block (Paper 10). This argument is pursued in the next paper by comparing the Doppler effect for sound and light. The conclusion is that the mechanisms are fundamentally different, and redshi ...
Doc - Paradigm Shift Now
... the strong force nor the EM force, almost oblivious to matter they pass right through it. They are harmless so the neutrino experiments require no shielding. They are the most common objects in the universe, outnumbering electrons or protons by a thousand million to one: the universe is a sea of Neu ...
... the strong force nor the EM force, almost oblivious to matter they pass right through it. They are harmless so the neutrino experiments require no shielding. They are the most common objects in the universe, outnumbering electrons or protons by a thousand million to one: the universe is a sea of Neu ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.